Figure 3.
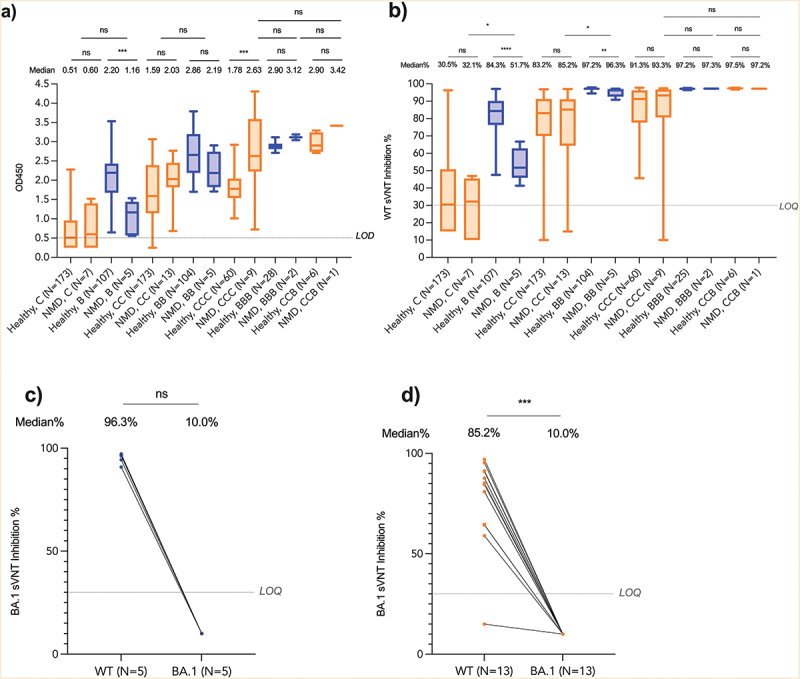
Immunogenicity for patients with neuromuscular diseases.
Antibody responses were determined before the first dose, second dose, 7–43 days after the second dose and 14–49 days after the third dose of BNT162b2 or CoronaVac in patients with neuromuscular diseases (NMDs) (n = 19) and healthy children and adolescents (n = 280). Three NMDs who had COVID-19 infection were excluded from the final analysis. ELISA=enzyme linked immunosorbent assay, LOQ=Limit of quantitation, Healthy=heathy children and adolescents, NMD=patients with neuromuscular diseases, sVNT=surrogate virus neutralization test, WT= wildtype SARS-CoV-2 virus. B, one dose of BNT162b2; BB, two doses of BNT162b2; C, one dose of CoronaVac; CC, two doses of CoronaVac; BBB, three doses of BNT162b2; CCC, three doses of CoronaVac; CCB, two doses of CoronaVac, and one dose of BNT162b2. BNT162b2 was represented as blue while CoronaVac was represented as orange color. Patients with NMDs using corticosteroids (deflazacort or prednisolone) daily were indicated as square dots in (c) & (d). *p < .05, **p < .01, ***p < .001, ****p < .0001 or NS (not significant).
