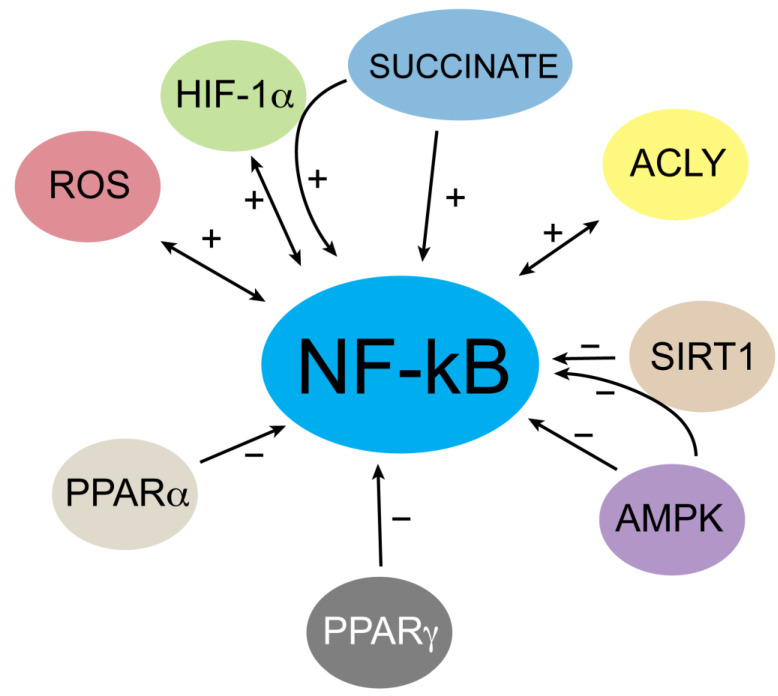
Figure 5.
Summary of the interplay between NF-κB and metabolic signals in innate immune cells. AMP-activated protein kinase (AMPK) negatively affects NF-κB activity dependent or independent of SIRT1. Through the hypoxia-inducible factor 1 subunit α (HIF-1α), increased levels of succinate act positively on NF-κB in M1 macrophages. Moreover, a solid crosstalk based on mutual regulation between HIF-1α and NF-κB occurs in innate immunity. NAD+-dependent SIRT1 lowers NF-κB transcriptional activity by p65 subunit deacetylation. ATP citrate lyase (ACLY) fosters p65 subunit acetylation, thus inducing NF-κB activation, which in turn upregulates ACLY human gene together with countless proinflammatory genes in M1 macrophages. Both the peroxisome proliferator-activated receptors PPARα and PPARγ suppress NF-κB signaling. Finally, a positive interaction takes place between ROS and NF-κB.