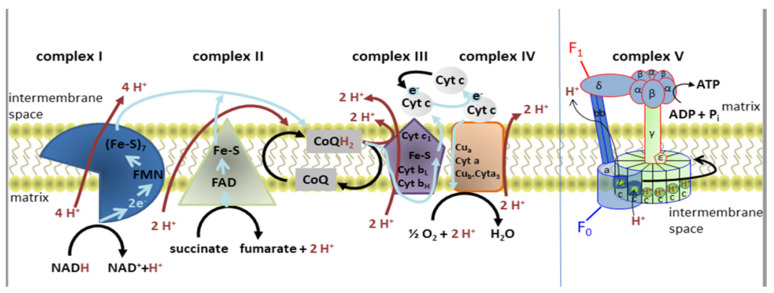
Figure 1.
Diagram of the electron transport chain and the structure of ATP synthase. Reduced nicotinamide adenine dinucleotide (NADH) donates electrons (blue arrows) that pass through complex I via flavin mononucleotide (FMN) and iron-sulfur (Fe-S) clusters. Together with two protons, they bind to oxidized coenzyme Q (CoQ) to form reduced coenzyme Q (CoQH2). This electron flow allows four H+ (red arrows) to be transported into the intermembrane space. Complex II is the electron side entrance, as electrons from succinate pass through oxidized flavin adenine dinucleotide (FAD+) and Fe-S before forming CoQH2. Electrons then flow from CoQH2 to complex III, and through cytochrome c (Cyt c) to complex IV. In total, ten H+ are transported into the intermembrane space for each NADH or six H+ for each FADH2. The stator (blue) and the rotor (green) form complex V. The FO domain of ATP synthase consists of three a subunits, three b subunits, and ten c subunits forming the c-ring. The a subunit contains the H+ ion half-channel, which is responsible for mediating proton movement across the membrane. The α and β subunits of F1 form a hexamer on the top of the γ subunit, which is inserted into the c-ring. Adapted from Ľupták et al. [3].