Abstract
In stage IV periodontitis patients with pathologic tooth migration (PTM), interdisciplinary treatment includes regenerative periodontal surgery (RPS) with an application of biomaterials and orthodontic therapy (OT) to restore function, esthetics and thereby quality of life (QoL). In a 24-month randomized trial we explored the synergy between regenerative medicine and biomechanical force application. The following methods were used: Forty-three patients had been randomized to a combined treatment comprising RPS and subsequent OT starting either 4 weeks (early OT) or 6 months (late OT) post-operatively. Clinical periodontal parameters and oral health-related QoL (GOHAI) were recorded up to 24 months. We obtained the following results: Mean clinical attachment gain (∆CAL ± SD) was significantly higher with early OT (5.96 ± 2.1 mm) versus late OT (4.65 ± 1.76 mm) (p = 0.034). Pocket closure (PPD ≤ 4 mm) was obtained in 91% of defects with early OT compared to 90% with late OT. GOHAI-scores decreased significantly from 26.1 ± 7.5 to 9.6 ± 4.7 (early OT) and 25.1 ± 7.1 to 12.7 ± 5.6 (late OT). Inconclusion, teeth severely compromised by intrabony defects and PTM can be treated successfully by RPS followed by early OT with the advantage of an overall reduced treatment time. As a result of the combined periodontal-orthodontic therapy, the oral health-related QoL of patients was significantly improved. Early stimulation of wound healing with orthodontic forces had a favorable impact on the outcomes of regenerative periodontal surgery.
Keywords: oral health-related quality of life, oral rehabilitation, regenerative periodontal therapy, orthodontic tooth movement, pathologic tooth migration, bovine bone mineral, collagen, enamel matrix derivative, stage IV periodontitis, randomized clinical trial
1. Introduction
In stage IV periodontitis patients with pathologic tooth migration (PTM), the sole treatment of periodontitis is usually not sufficient to restore oral health, correct masticatory dysfunction/malocclusion and improve their quality of life (QoL). The periodontal status of these patients is characterized by a similar severity and complexity in terms of inflammation, attachment and bone loss, as in stage III periodontitis, but may require a combined periodontal/orthodontic treatment (OT) for oral rehabilitation in order to restore function and esthetics [1,2,3,4].
It is well established that a regenerative periodontal treatment of intrabony defects can be successfully performed using various surgical procedures and biomaterials [5,6,7,8,9], provided that periodontal inflammation is under control by means of steps 1 and 2 of periodontal therapy. The combination of periodontal regenerative surgery (RPS) and consecutive orthodontic tooth movements in stage IV periodontitis patients was found to be efficient effective in the short-term [10], and the outcomes to be stable for up to 10 years under the premise of an adherence to a strict oral hygiene/maintenance protocol [11,12,13,14,15].
The optimal time interval between regenerative periodontal surgery and the initiation of OT has always been a matter of debate. Pini Prato and Chambrone (2020) [16] proposed waiting until the endpoint of regenerative therapy has been reached in order not to interfere with periodontal wound healing. In contrast, other authors have suggested a “stimulating” effect of early orthodontic tooth movement on the regenerative outcomes [17,18,19]. Several case reports [20,21,22,23,24], and a randomized clinical trial (RCT) [10], demonstrate that teeth severely compromised by intrabony defects and PTM can be treated successfully by regenerative surgery followed by early OT, with the advantage of an overall reduced treatment time.
Nevertheless, a gain in clinical attachment (CAL) and radiographic bone level, as well as reduction in probing pocket depths (PPD) and bleeding on probing (BOP) alone, may not be sufficient to evaluate the overall success of stage IV periodontitis treatment. Patient-related outcomes (PROMs) as “true endpoints” are reported to be equally or more relevant to patients’ daily lives [25,26] and it has been suggested that appropriate oral health-related quality of life (OHrQoL) outcomes should be included in the design of clinical studies [27]. Based on the rationale of a broader view of oral health and its rehabilitation, a number of tools have been introduced in order to measure the extent to which oral conditions affect an individual’s behavior and social life, as well as to complement the conventional clinical assessments of oral health [28,29,30,31,32,33].
Several studies reported that patients with more severe periodontitis rated their OHrQoL as poorer than those who had less severe periodontitis [29,34,35,36]. In addition, a positive perception by patients of the outcomes of long-term supportive therapy after regenerative surgery could be shown [37].
Malocclusion is an important and prevalent oral health problem worldwide [38,39] and has a negative impact on OHrQoL [40,41]. However, at present, there are no data from studies available that have evaluated the impact on OHrQoL in stage IV periodontitis with pathological tooth migration. Likewise, to the best of our knowledge, no studies have investigated the impact on OHrQoL of a combined periodontal and orthodontic treatment to restore function and esthetics in these patients [4].
A recent multi-center randomized trial [10] evaluated the periodontal outcomes of regenerative surgery in stage IV periodontitis patients in combination with staged orthodontic therapy after 12 months. Secondary outcomes of the study protocol also included OHrQoL measures at the baseline and up to 24 months. Here, we report the impact of the combined periodontal–orthodontic treatment on changes of clinical periodontal parameters and oral health-related quality of life.
2. Materials & Methods
2.1. Study Design and Patients
The present manuscript reports secondary outcomes of a prospective multicenter, multinational, randomized, parallel-group clinical trial (ClinicalTrials.gov, identifier: NCT 02761668) after 24 months. Special emphasis was given to patient-reported outcomes. The study protocol had been previously approved by the respective ethical committees for human subject trials from the centers participating in the study. The lead ethics committee was at the University of Bonn (code 034/16).
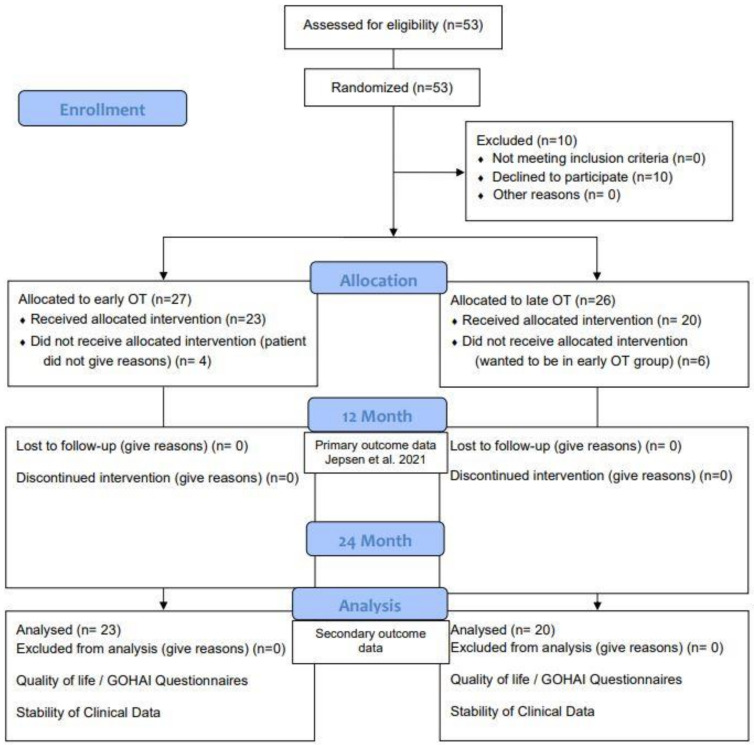
In brief, 43 patients with stage IV periodontitis were periodontally treated (steps 1–3 of periodontal therapy). Teeth with pathologic tooth migration and intrabony defects received regenerative periodontal surgery as described by Cortellini and Tonetti [5], followed by orthodontic treatment (OT) initiated 4 weeks after regenerative surgery (early OT, n = 23 patients) or 6 months after regenerative surgery (late OT, n = 20 patients) (Figure 1).
Figure 1.
Study flowchart following CONSORT (Consolidated Standards of Reporting Trials) guidelines for clinical trials. Fifty-three patients met the inclusion criteria, 26 patients were allocated to the group with late orthodontic therapy (OT) after regenerative periodontal surgery, and 27 to the group with early OT after regenerative periodontal surgery. A total of 10 patients withdrew from the study, 6 expected to be part of the early and withdrew after allocation to the late treatment group. The other 4 patients allocated to the test group did not want to continue the study without giving any reason. All patients of the study completed their 24-month examination.
The details of the study protocol were presented in a previous paper reporting 12-month clinical results [10].
2.2. Minimally Invasive Regenerative Periodontal Surgery
Microsurgical approaches, adapted to the treatment algorithm by Cortellini and Tonetti (2015), were applied to access the defects. A bone substitute (DBBMc, Bio Oss® Collagen; Geistlich, Wolhusen, Switzerland) was used to fill the defect and to prevent a soft tissue collapse. In non-contained defects, a collagen membrane (Bio Gide®Perio; Geistlich, Wolhusen, Switzerland) was applied. An enamel matrix derivative (EMD, Emdogain®; Straumann, Basel, Switzerland) was used for contained defects. Suturing was accomplished with non-resorbable 6-0 and 7-0 monofilament material (e-PTFE, W. L. Gore & Associates, Flagstaff, AZ, USA) by internal offset vertical mattress sutures, interrupted single sutures, double sling sutures, or a combination of these for achieving primary closure.
2.3. Orthodontic Therapy
Individual treatment objectives were defined and visualized for each subject. In cases of increased tooth mobility (>grade 1), passive fixed appliances were inserted prior to periodontal therapy for stabilization. Orthodontic tooth movement was carried out using fixed orthodontic appliances and individualized segmented arch mechanics in pre-adjusted 0.022-inch slot-sized brackets. Orthodontic movement was started with a 0.012 nickel–titanium (Ni–Ti) wire, followed by the alignment with the sequence of 0.014 Ni–Ti, 0.016 Ni–Ti, 0.018 Ni–Ti and 0.016 × 0.016 stainless steel wire. Up to the sequence of 0.016, Ni–Ti wire teeth were “secured” by a figure eight ligature in order to provide continuous transmission of orthodontic forces. Maximum emphasis was put on applying low forces and moments. Bone-borne temporary anchorage devices were used in some cases for anchorage reinforcement. Once treatment goals were achieved, orthodontic appliances were removed, and teeth were stabilized with bonded fixed retainers or fiber-reinforced restorations. In all cases, target teeth were moved toward the defect.
2.4. Supportive Care
Frequent recall visits were scheduled at 2 days, 2 weeks and 4 weeks post-surgery. Subsequently, all patients were enrolled in a regular supportive care program every 2 months for the duration of the study. In case of recurrent periodontal inflammation, OT was interrupted until inflammation could be controlled by gentle biofilm removal and oral hygiene reinforcement.
2.5. Periodontal Parameters
For each center, the same expert periodontists performed RPS, expert orthodontists performed OT and the same calibrated examiners collected all clinical parameters. In addition to the previously reported data at baseline, 6 and 12 months, the following periodontal outcome variables were recorded at 24 months:
-
(1)
Clinical attachment level (CAL),
-
(2)
Probing pocket depth (PPD),
-
(3)
Bleeding on probing (BOP),
-
(4)
Full-mouth bleeding scores (FMBS),
-
(5)
Full-mouth plaque scores (FMPS).
2.6. Patient-Reported Outcomes
After thorough explanation of the information to be collected, perceptions of OHrQoL were assessed with a questionnaire given to all participants regarding their oral status at baseline, 6, 12 and 24 months. The twelve-question general oral health assessment index (GOHAI), originally developed by Atchinson and Dolan [42], was used as a tool of measurement in validated translations of the GOHAI questionnaire into the native language of the participants [43,44,45]. Each of the twelve questions referred to their personal experience in the previous 3 months and was answered independently by the patient using a Likert scale (0 = “never” to 4 = “very often”) (Table S1).
For the evaluation, the answer scores for the twelve questions were summed up after coding [25], and for questions 3, 5 and 7, scoring was inverted and the scale thus ranged from 0–48 [28]. A high value indicates impairments of oral health-related quality of life, and low values indicate only a few problems.
2.7. Data Analysis
Computerized chairside periodontal data entry into a periodontal electronic database [Parostatus, Berlin, Germany or Florida Probe data base, USA] allowed for an export, via excel, into the statistical software program.
Descriptive statistics were summarized as means and standard deviations for quantitative data and frequencies and percentages for qualitative data. Means for each treatment group and differences between treatment groups were presented, along with associated 95% confidence intervals.
The comparison of clinical CAL changes after 24 months between treatment groups was based on a two-sided two-sample t-test, at the 5% level of significance. Statistical analysis of the clinical data was performed by an independent biostatistician (RF) using the software IBM® SPSS® Statistics 29 (Software version: 29.0.0).
3. Results
All 43 patients (mean age: 45.4 ± 11.9 years (early OT), 52.0 ± 9.4 years (late OT), 26 females, 17 males) were followed up until the time of their 24-month visit (until January 2022) and when they had completed all of their follow-up examinations (Figure 1). At 24 months, thirty patients had finished the combined treatment with 18 patients (78%) in the early group and 12 patients (60%) in the late group.
3.1. Periodontal Outcomes
Comparing the two treatment protocols, mean clinical attachment level gain (∆CAL ± SD) after 24 months was statistically significantly higher for early OT (5.96 ± 2.1; CI: 6.8, 5.1 mm) versus late OT (4.65 ± 1.76; CI: 5.4, 3.9 mm) (p = 0.034). When compared to 12 months, CAL showed further improvements at 24 months with an intergroup difference from the baseline of 1.31 mm in favor of early OT (Table 1).
Table 1.
Changes in clinical parameters CAL and PPD compared to baseline at 12 and 24 months (mean ± SD) for target sites in early and late treatment group. Differences between both groups in CAL change after 24 months (secondary outcome) were tested by unpaired t-test.
| Early OT n = 23 | Late OT n = 20 | Early vs. Late OT | |||||
|---|---|---|---|---|---|---|---|
| BL-12 mo | BL-24 mo | BL-12 mo | BL-24 mo | ∆Change BL-24 mo | |||
| ∆CAL (mean ± SD) | mm | 5.39 ± 2.2 | 5.96 ± 2.10 | 4.45 ± 1.7 | 4.65 ± 1.76 | 1.31 | p = 0.034 |
| Estimate | 95% CI | [6.3, 4.4] | [6.81, 5.10] | [5.3, 3.6] | [5.42, 3.88] | [2.5, 0.1] | |
| ∆PPD (mean ± SD) | mm | 4.21 ± 1.9 | 4.43 ± 1.62 | 3.90 ± 1.5 | 3.90 ± 1.33 | 0.53 | p = 0.248 |
| Estimate | 95% CI | [5.0, 3.4] | [5.1, 3.7] | [4.6, 3.2] | [4.5, 3.3] | [1.46, 0.39] | |
BL: Baseline, CAL: Clinical Attachment Level, PPD: Probing Pocket Depth, CI: Confidence Interval.
Table 2 depicts descriptive statistics for clinical parameters at baseline (BL), 12 and 24 months. Both groups were well-balanced at baseline with regard to CAL and PPD and showed statistically significant improved outcomes after 12 and 24 months (p < 0.0001): Baseline CAL had changed from 9.8 to 3.9 (early OT) and from 9.2 to 4.5 mm (late OT) at 24 months, respectively. Mean probing pocket depths (PPD) remained stable between 12 and 24 months with 2.9 mm (SD: 0.9) in the early OT group and 3.2 mm (SD: 0.9) in the late OT group. Pocket closure (PPD ≤ 4 mm) was obtained in 91% of defects with early OT compared to 90% with late OT. Over the course of treatment, patients maintained their good level of adherence to a strict 2-month performed supportive care protocol and full-mouth plaque scores were consistently low (FMPS well under 20%). This was accompanied by low full-mouth bleeding scores (FMBS) of 10.5 ± 4.8% vs. 12.7 ± 6.8% (early vs. late) at baseline, 10.6 ± 4.9% vs. 7.7 ± 4.9% at 6 months, 14.7 ± 13.1% vs. 11.3 ± 9.1% at 12 months and 9.2 ± 8.2% vs. 7.2 ± 4.1% at 24 months (Table 3).
Table 2.
Clinical parameters (mean ± SD) for target sites in early and late orthodontic treatment (OT) group at baseline, 12 months and 24 months. Differences between follow-up visits in CAL or PPD after 24 months (secondary outcome) were tested by paired t-test.
| Variable | Early OT (n = 23) | Late OT (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 12 mo | 24 mo | BL vs. 24 mo | Baseline | 12 mo | 24 mo | BL vs. 24 mo | ||
| CAL (mean ± SD) | mm | 9.8 ± 2.5 | 4.4 ± 1.7 | 3.9 ±1.9 | p <0.0001 | 9.2 ± 2.5 | 4.7 ± 2.4 | 4.50 ± 2.19 | p < 0.0001 |
| Estimate | 95% CI | [8.8, 10.9] | [3.7, 5.2] | [3.1, 4.7] | [5.0, 6.7] | [8.0, 10.4] | [3.6, 5.8] | [3.5, 5.5] | [3.9, 5.5] |
| PPD (mean ± SD) | mm | 7.3 ± 1.6 | 3.1 ± 0.9 | 2.9 ± 0.9 | p < 0.0001 | 7.1 ± 1.7 | 3.2 ± 1.1 | 3.2 ± 0.9 | p < 0.0001 |
| Estimate | 95% CI | [6.6, 8.0] | [2.7, 3.5] | [2.5, 3.3] | [3.7, 5.1] | [6.3, 7.9] | [2.7, 3.7] | [2.7, 3.6] | [3.1, 5.4] |
| PI | n (%) | 4 (17%) | 3 (13%) | 2 (8%) | 1 (5%) | 2 (10%) | 2 (10%) | ||
| BOP | n (%) | 13 (53%) | 7 (30%) | 4 (17%) | 9 (45%) | 3 (15%) | 0 (0%) | ||
| PUS | n (%) | 1 | 0 | 0 | 2 | 0 | 0 | ||
| Pocket closure (PPD ≤ 4 mm) | n (%) | n/a | 21 (91%) | 21 (91%) | n/a | 17 (85%) | 18 (90%) | ||
| Pocket closure (PPD ≤ 4 mm, no BOP) | n (%) | n/a | 16 (69%) | 18 (78%) | n/a | 15 (75%) | 15 (75%) | ||
BL: Baseline, CAL: Clinical Attachment Level, PPD: Probing Pocket Depth, PI: Plaque Index, BOP: Bleeding on Probing, PUS: Suppuration, CI: Confidence Interval.
Table 3.
Changes of patient-based plaque (FMPS) and bleeding scores (FMBS) over time: at baseline, 6, 12 and 24 months and numbers of patients in the different phases of orthodontic therapy (1 = active, 2 = retention phase, 3 = finished).
| Variables | Early OT (n = 23) | Late OT (n = 20) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 Months | 12 Months | 24 Months | Baseline | 6 Months | 12 Months | 24 Months | ||
| Orthodontic therapy | Number of patients | ||||||||
| active | n | 23 | 12 | 20 | 17 | 2 | |||
| retention | n | 9 | 5 | 3 | 6 | ||||
| finished | n | 2 | 18 | 0 | 12 | ||||
| Full-mouth plaque scores & bleeding scores |
|||||||||
| FMPS * (mean ± SD) | (%) | 12.9 ± 4.9 | 15.0 ± 6 | 16.9 ± 10.1 | 13.2 ± 7.3 | 15.2 ± 6.2 | 15.0 ± 7.0 | 17.1 ± 8.6 | 13.4 ± 6.7 |
| 95% CI | [11, 15] | [12, 18] | [13, 21] | [10, 16] | [12, 18] | [12, 18] | [13, 21] | [10, 16] | |
| FMBS ** (mean ± SD) | (%) | 10.5 ± 4.8 | 10.6 ± 4.9 | 14.7 ± 13.1 | 9.2 ± 8.2 | 12.7 ± 6.8 | 7.7 ± 4.9 | 11.0 ± 9.0 | 7.2 ± 4.1 |
| 95% CI | [8, 13] | [9, 13] | [9, 21] | [5, 13] | [10, 16] | [6, 10] | [7, 15] | [5, 9] | |
* FMPS = full-mouth plaque score [O’Leary-1972], ** FMBS = full-mouth bleeding score (out of 6 sites per tooth).
3.2. Oral Health-Related Quality of Life (OHrQoL)
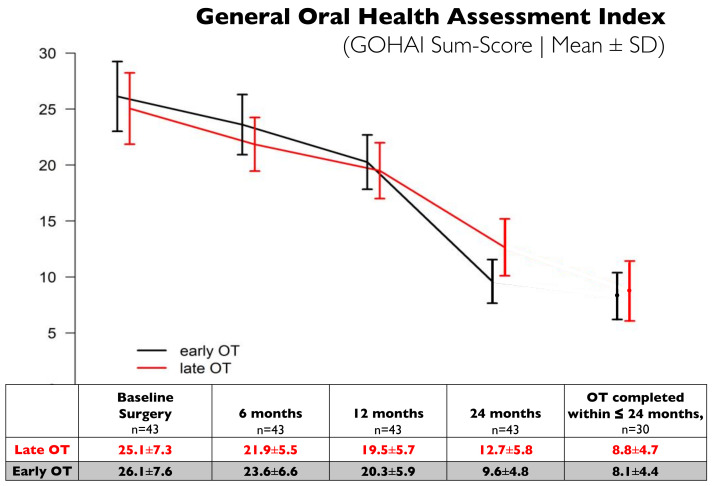
The OHrQoL of the patients, as measured by GOHAI sum-scores, improved continuously over the course of the study from 26.1 ± 7.5 to 9.6 ± 4.7 for early OT and from 25.1 ± 7.1 to 12.7 ± 5.6 for late OT (Figure 2) without relevant differences between the two treatment groups. In a subgroup of patients that had already completed the combined perio-ortho treatment at 24 months (n = 18/23 for early OT and n = 12/20 for late OT), the final GOHAI scores showed to be very similar (8.1 ± 4.4 vs. 8.8 ± 4.7).
Figure 2.
Patient-reported oral health-related quality of life (OHrQoL) as assessed by GOHAI sum-scores recorded at baseline (before surgery), at 6, 12, and 24 months. Mean GOHAI scores (± SD) in subjects (n = 43) treated with late OT (red line) and early OT (black line). Mean GOHAI scores (±SD) for subjects (n = 30) with OT completed in less or equal to 24 months are presented separately. GOHAI scores are expressed as total sum scores with all 12 questions included (values ranging from 0 to 48) with higher scores indicating greater negative impact on oral health-related quality of life [28].
4. Discussion
The results of this 24-month follow-up of a multicenter RCT provide evidence that early stimulation of periodontal wound healing with biomechanical forces has a favorable impact on the clinical outcomes of regenerative periodontal procedures in stage IV periodontitis patients with pathologic tooth migration. The present findings also support the hypothesis that a combined periodontal regenerative and orthodontic treatment could significantly improve the oral health-related quality of life of these patients. Both of the above findings are novel and of high clinical relevance.
The scientific rationale for our previous study [10] was the limited information available on the treatment of patients with stage IV periodontitis with intrabony defects and pathologic tooth migration in need of orthodontic therapy. The optimal interval between regenerative periodontal surgery and orthodontic therapy (OT) had been a matter of ongoing debate. The principal findings after 12 months were that significant periodontal improvements of a similar magnitude were observed following early (after 4 weeks) or late (after 6 months) initiation of OT.
So far, to the best of our knowledge, no other randomized clinical study has evaluated the effect of the timing of OT on periodontal outcomes over a period of 24 months. These findings confirm a suspected “stimulating” effect of early OT on regenerative outcomes [17,18,19,23]. Both healing after RPS and healing after OT are highly coordinated processes in which various cells, such as immune, bone and periodontal ligament (PDL) cells, cytokines and signals/pathways, are involved. The whole periodontal attachment apparatus, including the alveolar bone, exhibits biological responses and changes, including a modification of the local vascularization. As known from wound healing studies, cells can respond to mechanical signals and micromechanical forces, where micro-deformations on the cellular level can stimulate cell proliferation and division [46]. In particular, PDL fibroblasts are mechano-sensing cells responsible for a complex immune response associated with the initiation of bone remodeling [47]. Fibroblasts can react to micro-deformational forces with increased proliferation and expression of collagen type I, basic fibroblast growth factor and transforming growth factor beta [48]. Mechanosensitive cells of the periodontium possess the ability to respond to a mechanical load by changing their cellular functions, including, among others, cytoskeletal rearrangement [47,49,50]. Mechanical stress of an appropriate amount which is applied to the cell membrane is detected, among others, by integrins and focal adhesion molecules, and, in this way, it triggers the assembly of specified “stress fibers” of the cytoskeleton. The latter is connected to the nuclear lamina by the linker of nucleoskeleton and cytoskeleton (LINC) complex [51]. In this way, the rearrangement of the cytoskeleton is transduced to the nucleus, leading to transcriptional changes affecting pathways that regulate cell proliferation, differentiation, motility, as well as the production of cytokines and growth factors [52].
In periodontal disease [stage III or IV], the wound associated with intrabony periodontal defects remains in the inflammatory phase and fibroblasts cannot perform their tasks due to the inflammatory environment. It is known that, in the initial phase of tooth movement, mechanical forces distort the interstitial space within the PDL and alveolar bone [53,54]. By application of micro-mechanical forces applied shortly after regenerative surgery, wound micro-deformations may induce cellular proliferation and migration [46] and enhance periodontal regeneration as well as tissue remodeling [55]. However, it has to be realized that the biological mechanisms underlying orthodontic tooth movement are still not fully understood [56]. More well-designed preclinical experiments have to be performed in order to elucidate the synergy between regenerative medicine and biomechanical force application in periodontal defects to explain the favorable clinical outcomes of the present study.
To the best of our knowledge, no other prospective study has investigated the impact of a combined periodontal–orthodontic therapy on the quality of life of patients with stage IV periodontitis affected by pathologic tooth migration. The finding of significant improvements in OHrQoL, as measured by a continuous reduction in GOHAI scores, confirms that the combined treatment not only improved the objectively assessed periodontal conditions of the patients but also their subjective perception of regained oral health, due to improved esthetics and function.
Earlier studies have already demonstrated that periodontal therapy has a positive impact on OHrQoL in patients affected by periodontitis, as measured by various accepted scoring systems [57,58,59,60]. These effects were mainly reported between 1 week and up to 12 months following the non-surgical periodontal treatment. No significant differences in the positive impact on OHrQoL were seen when comparing quadrant-wise scaling and root planing versus one-stage full-mouth disinfection [61]. Patients treated by periodontal surgery reported a worse OHrQoL in the first post-operative week [62]. With regard to the impact of periodontal surgery compared to non-surgical treatment, in some studies a low impact was observed [58,63,64,65] whereas others reported more pronounced additional improvements following surgery [66]. It has also been shown that orthodontic therapy has a positive impact on OHrQoL in patients affected by malpositioned teeth/malocclusion. Most of these studies, however, were conducted in children and adolescents [67]. Little, if any, information is available on adults [68]. Based on these reports of positive impacts on OHrQoL of orthodontic therapy in patients with malocclusion and of periodontal therapy in patients with periodontitis it can be assumed that the significant improvements observed in the present study can be attributed to both components of the combined therapy.
GOHAI sum scores, in the way they were calculated in the present study, can range between 48 (worst OHrQoL) and 0 [28,69]. The GOHAI rather than the alternative OHIP scoring system was chosen for the present study based on the findings by Öhrn and Jönsson [28]. With regard to the magnitude of the effect observed, already at 12 months after periodontal surgery mean GOHAI scores were reduced by about 6 points and decreased by another 7 (late OT) and 10 points (early OT) at 24 months, respectively. Interestingly, in the subgroup of patients in whom orthodontic treatment was completed at 24 months, the mean GOHAI scores were below 9, with no difference between the groups. In the absence of a meaningful benchmark for comparison between mean scores, Tsakos [70] proposed to employ minimally important differences (MID) instead to assist data interpretability. Following this suggestion, Jönsson and Öhrn [69] established a MID of 3 for improvements in GOHAI scores in 87 patients before, and at 12 months after, non-surgical periodontal therapy.
At present, no such MID values have been reported for the treatment of stage IV periodontitis patients. However, the significant improvements in OHrQoL (>15 GOHAI score points) in the present study compare favorably with a reduction of 5.4 points, as reported in a recent systematic review with meta-analysis, following prosthetic rehabilitation of fully/partially edentulous with previous periodontitis [71].
The present 24-month follow-up of an RCT has several strengths, such as the prospective well-controlled design, the length of follow-up, the high adherence of the patients to supportive care and compliance with a high level of self-performed oral hygiene, and the multi-center, multi-national approach, among others. On the other hand, the limited number of subjects and the fact that patients were treated in specialist settings may limit the generalizability of the results.
Future interdisciplinary studies with close cooperation between periodontics and orthodontics are warranted. A further exploration of the synergy between regenerative periodontal medicine and biomechanical orthodontic forces and their impact on the patients’ OHrQoL is of high clinical relevance because it is well-known that many adult patients affected by severe periodontitis are interested in seeking orthodontic treatment for oral rehabilitation due to the esthetic and functional changes caused by pathologic tooth migration [72].
5. Conclusions
Within the limitations of this study, taken together, the findings of the present 24-month follow-up show that a combined periodontal regenerative and orthodontic treatment for patients with good adherence to supportive care protocols resulted in:
-
(1)
significantly improved periodontal conditions,
-
(2)
significantly higher CAL-gain for early initiation of OT,
-
(3)
an overall significantly improved OHrQoL.
From the perspective of oral rehabilitation, orthodontic therapy plays an important role in the comprehensive treatment of stage IV periodontitis patients. More well-designed preclinical studies are warranted to further elucidate the mechanisms underlying the observed synergy between periodontal regenerative medicine and orthodontic biomechanical force application in advanced periodontitis.
Acknowledgments
The authors thank Philipp Skora and Sven Wenzel for their assistance during follow-up visits and supportive periodontal care. We also thank the Osteology Foundation for the support with Geistlich biomaterials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bioengineering10060695/s1, Table S1: Questionnaire for patient reported outcomes (impact on patients’ OHrQoL).
Author Contributions
Funding Acquisition: K.J. and S.J.; Conceptualization: K.J. and S.J. contributed to the conception, methodology and design of the study; Project Administration: K.J., C.T., D.C., C.M. and S.J.; authors K.J., C.T., E.K., P.W., D.C., L.G., A.J., I.S.-S. and C.M. contributed to the clinical phases of the study and data acquisition; R.F. designed the statistical methodology, data curation and formal analysis; K.J., C.T. and S.J. contributed to interpretation of the data and drafted and finalized the manuscript. All authors have critically reviewed, substantially revised and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee, University of Bonn (code 034/16) for the centers Bonn and Aachen and by the competent local authorities for the centers in Torino (code PROT 04-2017) and Madrid (code 16/492-E).
Informed Consent Statement
Written informed consent for publication was obtained from all participating patients involved in the study and to publish this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest with regard to this study.
Funding Statement
This study was partially funded by an advanced researcher grant from the Osteology Foundation (Grant Project No. 15-249) at the University of Bonn (Funding Acquisition: K.J., S.J.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Martin C., Celis B., Ambrosio N., Bollain J., Antonoglou G.N., Figuero E. Effect of orthodontic therapy in periodontitis and non-periodontitis patients: A systematic review with meta-analysis. J. Clin. Periodontol. 2022;49((Suppl. S24)):72–101. doi: 10.1111/jcpe.13487. [DOI] [PubMed] [Google Scholar]
- 2.Papageorgiou S.N., Antonoglou G.N., Michelogiannakis D., Kakali L., Eliades T., Madianos P. Effect of periodontal-orthodontic treatment of teeth with pathological tooth flaring, drifting, and elongation in patients with severe periodontitis: A systematic review with meta-analysis. J. Clin. Periodontol. 2022;49((Suppl. S24)):102–120. doi: 10.1111/jcpe.13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kloukos D., Roccuzzo A., Stähli A., Sculean A., Katsaros C., Salvi G. Effect of combined periodontal and orthodontic treatment of tilted molars, and of teeth with intrabony and furcation defects in stage IV periodontitis patients. A systematic review. J. Clin. Periodontol. 2022;49((Suppl. S24)):121–148. doi: 10.1111/jcpe.13509. [DOI] [PubMed] [Google Scholar]
- 4.Herrera D., Sanz M., Kebschull M., Jepsen S., Sculean A., Berglundh T., Papapanou P.N., Chapple I., Tonetti M.S., Participants E.F.P.W., et al. Treatment of stage IV periodontitis: The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2022;49((Suppl. S24)):4–71. doi: 10.1111/jcpe.13639. [DOI] [PubMed] [Google Scholar]
- 5.Cortellini P., Tonetti M.S. Clinical concepts for regenerative therapy in intrabony defects. Periodontol. 2000. 2015;68:282–307. doi: 10.1111/prd.12048. [DOI] [PubMed] [Google Scholar]
- 6.Broseler F., Tietmann C., Hinz A.K., Jepsen S. Long-term results of periodontal regenerative therapy: A retrospective practice-based cohort study. J. Clin. Periodontol. 2017;44:520–529. doi: 10.1111/jcpe.12723. [DOI] [PubMed] [Google Scholar]
- 7.Nibali L., Koidou V.P., Nieri M., Barbato L., Pagliaro U., Cairo F. Regenerative surgery versus access flap for the treatment of intra-bony periodontal defects: A systematic review and meta-analysis. J. Clin. Periodontol. 2020;47((Suppl. S22)):320–351. doi: 10.1111/jcpe.13237. [DOI] [PubMed] [Google Scholar]
- 8.Nibali L., Sultan D., Arena C., Pelekos G., Lin G.H., Tonetti M. Periodontal infrabony defects: Systematic review of healing by defect morphology following regenerative surgery. J. Clin. Periodontol. 2021;48:100–113. doi: 10.1111/jcpe.13381. [DOI] [PubMed] [Google Scholar]
- 9.Sanz M., Herrera D., Kebschull M., Chapple I., Jepsen S., Berglundh T., Sculean A., Tonetti M.S., Participants E.F.P.W., Methodological C. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020;47:4–60. doi: 10.1111/jcpe.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jepsen K., Tietmann C., Kutschera E., Wullenweber P., Jager A., Cardaropoli D., Gaveglio L., Sanz Sanchez I., Martin C., Fimmers R., et al. The effect of timing of orthodontic therapy on the outcomes of regenerative periodontal surgery in patients with stage IV periodontitis: A multicenter randomized trial. J. Clin. Periodontol. 2021;48:1282–1292. doi: 10.1111/jcpe.13528. [DOI] [PubMed] [Google Scholar]
- 11.Roccuzzo M., Marchese S., Dalmasso P., Roccuzzo A. Periodontal Regeneration and Orthodontic Treatment of Severely Periodontally Compromised Teeth: 10-Year Results of a Prospective Study. Int. J. Periodontics Restor. Dent. 2018;38:801–809. doi: 10.11607/prd.3756. [DOI] [PubMed] [Google Scholar]
- 12.Aimetti M., Garbo D., Ercoli E., Grigorie M.M., Citterio F., Romano F. Long-Term Prognosis of Severely Compromised Teeth Following Combined Periodontal and Orthodontic Treatment: A Retrospective Study. Int. J. Periodontics Restor. Dent. 2020;40:95–102. doi: 10.11607/prd.4523. [DOI] [PubMed] [Google Scholar]
- 13.Tietmann C., Broseler F., Axelrad T., Jepsen K., Jepsen S. Regenerative periodontal surgery and orthodontic tooth movement in stage IV periodontitis: A retrospective practice-based cohort study. J. Clin. Periodontol. 2021;48:668–678. doi: 10.1111/jcpe.13442. [DOI] [PubMed] [Google Scholar]
- 14.Garbo D., Aimetti M., Bongiovanni L., Vidotto C., Mariani G.M., Baima G., Romano F. Periodontal and Orthodontic Synergy in the Management of Stage IV Periodontitis: Challenges, Indications and Limits. Life. 2022;12:2131. doi: 10.3390/life12122131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tietmann C., Jepsen S., Heibrok H., Wenzel S., Jepsen K. Long-term stability of regenerative periodontal surgery and orthodontic tooth movement in stage IV periodontitis: 10-year data of a retrospective study. J. Periodontol. 2023 doi: 10.1002/JPER.23-0081. [DOI] [PubMed] [Google Scholar]
- 16.Pini Prato G.P., Chambrone L. Orthodontic treatment in periodontal patients: The use of periodontal gold standards to overcome the “grey zone”. J. Periodontol. 2020;91:437–441. doi: 10.1002/JPER.19-0306. [DOI] [PubMed] [Google Scholar]
- 17.Nemcovsky C.E., Sasson M., Beny L., Weinreb M., Vardimon A.D. Periodontal healing following orthodontic movement of rat molars with intact versus damaged periodontia towards a bony defect. Eur. J. Orthod. 2007;29:338–344. doi: 10.1093/ejo/cjm015. [DOI] [PubMed] [Google Scholar]
- 18.Vardimon A.D., Nemcovsky C.E., Dre E. Orthodontic tooth movement enhances bone healing of surgical bony defects in rats. J. Periodontol. 2001;72:858–864. doi: 10.1902/jop.2001.72.7.858. [DOI] [PubMed] [Google Scholar]
- 19.Diedrich P., Fritz U., Kinzinger G., Angelakis J. Movement of periodontally affected teeth after guided tissue regeneration (GTR)-an experimental pilot study in animals. J. Orofac. Orthop. 2003;64:214–227. doi: 10.1007/s00056-003-0240-8. [DOI] [PubMed] [Google Scholar]
- 20.Cardaropoli D., Re S., Manuzzi W., Gaveglio L., Cardaropoli G. Bio-oss collagen and orthodontic movement for the treatment of infrabony defects in the esthetic zone. Int. J. Periodont. Rest. 2006;26:553–559. [PubMed] [Google Scholar]
- 21.Ogihara S., Wang H.L. Periodontal regeneration with or without limited orthodontics for the treatment of 2- or 3-wall infrabony defects. J. Periodontol. 2010;81:1734–1742. doi: 10.1902/jop.2010.100127. [DOI] [PubMed] [Google Scholar]
- 22.Attia M.S., Hazzaa H.H., Al-Aziz F.A., Elewa G.M. Evaluation of Adjunctive Use of Low-Level Diode Laser Biostimulation with Combined Orthodontic Regenerative Therapy. J. Int. Acad. Periodontol. 2019;21:63–73. [PubMed] [Google Scholar]
- 23.Attia M.S., Shoreibah E.A., Ibrahim S.A., Nassar H.A. Regenerative Therapy of Osseous Defects Combined with Orthodontic Tooth Movement. J. Int. Acad. Periodontol. 2012;14:17–25. [PubMed] [Google Scholar]
- 24.Ghezzi C., Viganò V.M., Francinetti P., Zanotti G., Masiero S. Orthodontic Treatment After Induced Periodontal Regeneration in Deep Infrabony Defects. Clin. Adv. Periodontics. 2013;3:24–31. doi: 10.1902/cap.2012.110085. [DOI] [Google Scholar]
- 25.Locker D., Matear D., Stephens M., Lawrence H., Payne B. Comparison of the GOHAI and OHIP-14 as measures of the oral health-related quality of life of the elderly. Community Dent. Oral Epidemiol. 2008;29:373–381. doi: 10.1111/j.1600-0528.2001.290507.x. [DOI] [PubMed] [Google Scholar]
- 26.FDI General Assembly Oral Health and Quality of Life. [(accessed on 22 April 2023)]. Available online: https://www.fdiworlddental.org/oral-health-and-quality-life.
- 27.Graziani F., Tsakos G. Patient-based outcomes and quality of life. Periodontol. 2000. 2020;83:277–294. doi: 10.1111/prd.12305. [DOI] [PubMed] [Google Scholar]
- 28.Ohrn K., Jonsson B. A comparison of two questionnaires measuring oral health-related quality of life before and after dental hygiene treatment in patients with periodontal disease. Int. J. Dent. Hyg. 2012;10:9–14. doi: 10.1111/j.1601-5037.2011.00511.x. [DOI] [PubMed] [Google Scholar]
- 29.Needleman I., McGrath C., Floyd P., Biddle A. Impact of oral health on the life quality of periodontal patients. J. Clin. Periodontol. 2004;31:454–457. doi: 10.1111/j.1600-051X.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 30.Yang C., Crystal Y.O., Ruff R.R., Veitz-Keenan A., McGowan R.C., Niederman R. Quality Appraisal of Child Oral Health-Related Quality of Life Measures: A Scoping Review. JDR Clin. Trans. Res. 2020;5:109–117. doi: 10.1177/2380084419855636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schierz O., Baba K., Fueki K. Functional oral health-related quality of life impact: A systematic review in populations with tooth loss. J. Oral Rehabil. 2021;48:256–270. doi: 10.1111/joor.12984. [DOI] [PubMed] [Google Scholar]
- 32.Larsson P. Methodological studies of orofacial aesthetics, orofacial function and oral health-related quality of life. Swed. Dent. J. Suppl. 2010;204:11–98. [PubMed] [Google Scholar]
- 33.McGrath C., Bedi R. An evaluation of a new measure of oral health related quality of life—OHQoL-UK(W) Community Dent. Health. 2001;18:138–143. [PubMed] [Google Scholar]
- 34.Aslund M., Pjetursson B.E., Lang N.P. Measuring oral health-related quality-of-life using OHQoL-GE in periodontal patients presenting at the University of Berne, Switzerland. Oral Health Prev. Dent. 2008;6:191–197. [PubMed] [Google Scholar]
- 35.Ferreira M.C., Dias-Pereira A.C., Branco-de-Almeida L.S., Martins C.C., Paiva S.M. Impact of periodontal disease on quality of life: A systematic review. J. Periodontal Res. 2017;52:651–665. doi: 10.1111/jre.12436. [DOI] [PubMed] [Google Scholar]
- 36.Buset S.L., Walter C., Friedmann A., Weiger R., Borgnakke W.S., Zitzmann N.U. Are periodontal diseases really silent? A systematic review of their effect on quality of life. J. Clin. Periodontol. 2016;43:333–344. doi: 10.1111/jcpe.12517. [DOI] [PubMed] [Google Scholar]
- 37.Franke M., Broseler F., Tietmann C. Patient-related evaluation after systematic periodontal therapy—A clinical study on periodontal health-related quality of life (PHQoL) Oral Health Prev. Dent. 2015;13:163–168. doi: 10.3290/j.ohpd.a32340. [DOI] [PubMed] [Google Scholar]
- 38.Petersen P.E. The World Oral Health Report 2003: Continuous improvement of oral health in the 21st century—The approach of the WHO Global Oral Health Programme. Community Dent. Oral Epidemiol. 2003;31((Suppl. S1)):3–23. doi: 10.1046/j..2003.com122.x. [DOI] [PubMed] [Google Scholar]
- 39.Petersen P.E. Global policy for improvement of oral health in the 21st century-implications to oral health research of World Health Assembly 2007, World Health Organization. Community Dent. Oral Epidemiol. 2009;37:1–8. doi: 10.1111/j.1600-0528.2008.00448.x. [DOI] [PubMed] [Google Scholar]
- 40.Ajwa N., AlHammad A., AlAmmar L., AlMarjan M., AlShugair T., AlManie L., Bangalore D. The Influence of Orthodontic Treatment Need on Oral Health-Related Quality of Life among 12–18-Year-Old Adolescents in Riyadh. Healthcare. 2022;10:2153. doi: 10.3390/healthcare10112153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bekes K., Kuhr K., Ohm C., Frenzel Baudisch N., Jordan A.R. Does orthodontic treatment need have an impact on oral health-related quality of life? J. Orofac. Orthop. 2023;84:19–25. doi: 10.1007/s00056-022-00438-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Atchison K.A., Dolan T.A. Development of the Geriatric Oral Health Assessment Index. J. Dent. Educ. 1990;54:680–687. doi: 10.1002/j.0022-0337.1990.54.11.tb02481.x. [DOI] [PubMed] [Google Scholar]
- 43.Hassel A.J., Rolko C., Koke U., Leisen J., Rammelsberg P. A German version of the GOHAI. Community Dent. Oral Epidemiol. 2008;36:34–42. doi: 10.1111/j.1600-0528.2007.00351.x. [DOI] [PubMed] [Google Scholar]
- 44.Aguirre-Bustamante J., Baron-Lopez F.J., Carmona-Gonzalez F.J., Perez-Farinos N., Warnberg J. Validation of a modified version of the Spanish Geriatric Oral Health Assessment Index (GOHAI-SP) for adults and elder people. BMC Oral Health. 2020;20:61. doi: 10.1186/s12903-020-1047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bianco A., Mazzea S., Fortunato L., Giudice A., Papadopoli R., Nobile C.G.A., Pavia M. Oral Health Status and the Impact on Oral Health-Related Quality of Life among the Institutionalized Elderly Population: A Cross-Sectional Study in an Area of Southern Italy. Int. J. Environ. Res. Public Health. 2021;18:2175. doi: 10.3390/ijerph18042175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wiegand C., White R. Microdeformation in wound healing. Wound Repair Regen. 2013;21:793–799. doi: 10.1111/wrr.12111. [DOI] [PubMed] [Google Scholar]
- 47.Krishnan V., Davidovitch Z. On a path to unfolding the biological mechanisms of orthodontic tooth movement. J. Dent. Res. 2009;88:597–608. doi: 10.1177/0022034509338914. [DOI] [PubMed] [Google Scholar]
- 48.Lu F., Ogawa R., Nguyen D.T., Chen B., Guo D., Helm D.L., Zhan Q., Murphy G.F., Orgill D.P. Microdeformation of three-dimensional cultured fibroblasts induces gene expression and morphological changes. Ann. Plast. Surg. 2011;66:296–300. doi: 10.1097/SAP.0b013e3181ea1e9b. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki R., Nemoto E., Shimauchi H. Cyclic tensile force up-regulates BMP-2 expression through MAP kinase and COX-2/PGE2 signaling pathways in human periodontal ligament cells. Exp. Cell Res. 2014;323:232–241. doi: 10.1016/j.yexcr.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Tantilertanant Y., Niyompanich J., Everts V., Supaphol P., Pavasant P., Sanchavanakit N. Cyclic tensile force stimulates BMP9 synthesis and in vitro mineralization by human periodontal ligament cells. J. Cell Physiol. 2019;234:4528–4539. doi: 10.1002/jcp.27257. [DOI] [PubMed] [Google Scholar]
- 51.Fletcher D.A., Mullins R.D. Cell mechanics and the cytoskeleton. Nature. 2010;463:485–492. doi: 10.1038/nature08908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maurer M., Lammerding J. The Driving Force: Nuclear Mechanotransduction in Cellular Function, Fate, and Disease. Annu. Rev. Biomed. Eng. 2019;21:443–468. doi: 10.1146/annurev-bioeng-060418-052139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y., Zhan Q., Bao M., Yi J., Li Y. Biomechanical and biological responses of periodontium in orthodontic tooth movement: Up-date in a new decade. Int. J. Oral Sci. 2021;13:20. doi: 10.1038/s41368-021-00125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zainal Ariffin S.H., Yamamoto Z., Zainol Abidin I.Z., Megat Abdul Wahab R., Zainal Ariffin Z. Cellular and molecular changes in orthodontic tooth movement. Sci. World J. 2011;11:1788–1803. doi: 10.1100/2011/761768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vining K.H., Mooney D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017;18:728–742. doi: 10.1038/nrm.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alghamdi B., Jeon H.H., Ni J., Qiu D., Liu A., Hong J.J., Ali M., Wang A., Troka M., Graves D.T. Osteoimmunology in Periodontitis and Orthodontic Tooth Movement. Curr. Osteoporos. Rep. 2023;21:128–146. doi: 10.1007/s11914-023-00774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wong R.M., Ng S.K., Corbet E.F., Keung Leung W. Non-surgical periodontal therapy improves oral health-related quality of life. J. Clin. Periodontol. 2012;39:53–61. doi: 10.1111/j.1600-051X.2011.01797.x. [DOI] [PubMed] [Google Scholar]
- 58.Shanbhag S., Dahiya M., Croucher R. The impact of periodontal therapy on oral health-related quality of life in adults: A systematic review. J. Clin. Periodontol. 2012;39:725–735. doi: 10.1111/j.1600-051X.2012.01910.x. [DOI] [PubMed] [Google Scholar]
- 59.Brauchle F., Noack M., Reich E. Impact of periodontal disease and periodontal therapy on oral health-related quality of life. Int. Dent. J. 2013;63:306–311. doi: 10.1111/idj.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Botelho J., Machado V., Proenca L., Bellini D.H., Chambrone L., Alcoforado G., Mendes J.J. The impact of nonsurgical periodontal treatment on oral health-related quality of life: A systematic review and meta-analysis. Clin. Oral Investig. 2020;24:585–596. doi: 10.1007/s00784-019-03188-1. [DOI] [PubMed] [Google Scholar]
- 61.Santuchi C.C., Cortelli J.R., Cortelli S.C., Cota L.O., Fonseca D.C., Alencar C.O., Costa F.O. Scaling and Root Planing per Quadrant Versus One-Stage Full-Mouth Disinfection: Assessment of the Impact of Chronic Periodontitis Treatment on Quality of Life—A Clinical Randomized, Controlled Trial. J. Periodontol. 2016;87:114–123. doi: 10.1902/jop.2015.150105. [DOI] [PubMed] [Google Scholar]
- 62.Ozcelik O., Haytac M.C., Seydaoglu G. Immediate post-operative effects of different periodontal treatment modalities on oral health-related quality of life: A randomized clinical trial. J. Clin. Periodontol. 2007;34:788–796. doi: 10.1111/j.1600-051X.2007.01120.x. [DOI] [PubMed] [Google Scholar]
- 63.Saito A., Hosaka Y., Kikuchi M., Akamatsu M., Fukaya C., Matsumoto S., Ueshima F., Hayakawa H., Fujinami K., Nakagawa T. Effect of initial periodontal therapy on oral health-related quality of life in patients with periodontitis in Japan. J. Periodontol. 2010;81:1001–1009. doi: 10.1902/jop.2010.090663. [DOI] [PubMed] [Google Scholar]
- 64.Saito A., Ota K., Hosaka Y., Akamatsu M., Hayakawa H., Fukaya C., Ida A., Fujinami K., Sugito H., Nakagawa T. Potential impact of surgical periodontal therapy on oral health-related quality of life in patients with periodontitis: A pilot study. J. Clin. Periodontol. 2011;38:1115–1121. doi: 10.1111/j.1600-051X.2011.01796.x. [DOI] [PubMed] [Google Scholar]
- 65.Theodoridis C., Violesti A., Nikiforidou M., Menexes G.C., Vouros I.D. Short-Term Impact of Non-Surgical and Surgical Periodontal Therapy on Oral Health-Related Quality of Life in a Greek Population-A Prospective Cohort Study. Dent. J. 2020;8:54. doi: 10.3390/dj8020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Makino-Oi A., Ishii Y., Hoshino T., Okubo N., Sugito H., Hosaka Y., Fukaya C., Nakagawa T., Saito A. Effect of periodontal surgery on oral health-related quality of life in patients who have completed initial periodontal therapy. J. Periodontal Res. 2016;51:212–220. doi: 10.1111/jre.12300. [DOI] [PubMed] [Google Scholar]
- 67.Ribeiro L.G., Antunes L.S., Kuchler E.C., Baratto-Filho F., Kirschneck C., Guimaraes L.S., Antunes L.A.A. Impact of malocclusion treatments on Oral Health-Related Quality of Life: An overview of systematic reviews. Clin. Oral Investig. 2023;27:907–932. doi: 10.1007/s00784-022-04837-8. [DOI] [PubMed] [Google Scholar]
- 68.Kara-Boulad J.M., Burhan A.S., Hajeer M.Y., Khattab T.Z., Nawaya F.R. Evaluation of the Oral Health-Related Quality of Life (OHRQoL) in Patients Undergoing Lingual Versus Labial Fixed Orthodontic Appliances: A Randomized Controlled Clinical Trial. Cureus. 2022;14:e23379. doi: 10.7759/cureus.23379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jonsson B., Ohrn K. Evaluation of the effect of non-surgical periodontal treatment on oral health-related quality of life: Estimation of minimal important differences 1 year after treatment. J. Clin. Periodontol. 2014;41:275–282. doi: 10.1111/jcpe.12202. [DOI] [PubMed] [Google Scholar]
- 70.Tsakos G., Allen P.F., Steele J.G., Locker D. Interpreting oral health-related quality of life data. Community Dent. Oral Epidemiol. 2012;40:193–200. doi: 10.1111/j.1600-0528.2011.00651.x. [DOI] [PubMed] [Google Scholar]
- 71.Gennai S., Izzetti R., Pioli M.C., Music L., Graziani F. Impact of rehabilitation versus edentulism on systemic health and quality of life in patients affected by periodontitis: A systematic review and meta-analysis. J. Clin. Periodontol. 2022;49((Suppl. S24)):328–358. doi: 10.1111/jcpe.13526. [DOI] [PubMed] [Google Scholar]
- 72.Hirschfeld J., Reichardt E., Sharma P., Hilber A., Meyer-Marcotty P., Stellzig-Eisenhauer A., Schlagenhauf U., Sickel F.E. Interest in orthodontic tooth alignment in adult patients affected by periodontitis: A questionnaire-based cross-sectional pilot study. J. Periodontol. 2019;90:957–965. doi: 10.1002/JPER.18-0578. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.