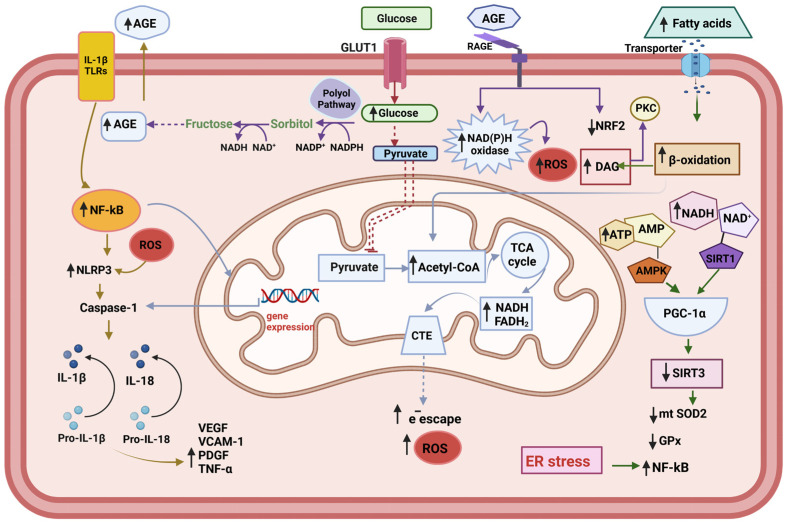
Figure 1.
Metabolic changes in the endothelial cell. The endogenous increased cytokines act through the Toll-like receptors (TLRs) to activate NF-kB—NLRP3 inflammasome pathway. Under conditions of hyperglycemia, the accumulation of reducing equivalents (NADH+H, FADH2) feeds the respiratory chain with electrons, which leads to greater electron escape and increased superoxide radical (O2−•) production. The increment of polyol pathway leads to Advanced glycation end products (AGEs) accumulation, which act on the AGEs receptors (RAGE), leading to the activation of pro-oxidative enzymes, such as NAD(P)H oxidase, and decrement of NRF2, one important component of the anti-oxidative system of the cell. Increased β-oxidation upon the excess of fatty acid contributes to the accumulation of D-acyl glycerol, thus conducing to the activation of protein kinase C (PKC). The translocation of NF-κB to the nucleus activates the transcription of its target genes, including pro-IL-1, pro-il-18, and Pro-Caspase 1. The activation of NLRP3 inflammasome facilities the cleavage of the pro-Caspase 1 into its active form Caspase1, which in turn transforms IL-1β and IL-18 into their active forms. Subsequently, IL-18 induces the production of TNF-λ, which in turn promotes the synthesis and release of IL-6 and C reactive protein (CRP) (not shown). At the same time, the production of adhesion molecules is activated: vascular adhesion molecule-1 (VCAM-1), platelet-derived grown factor (PDGF), and vascular endothelial grown factor (VEGF). The endoplasmic reticulum (ER) stress contributes to the trigger for NLRP3 inflammasome activation and potentiating oxidative stress.