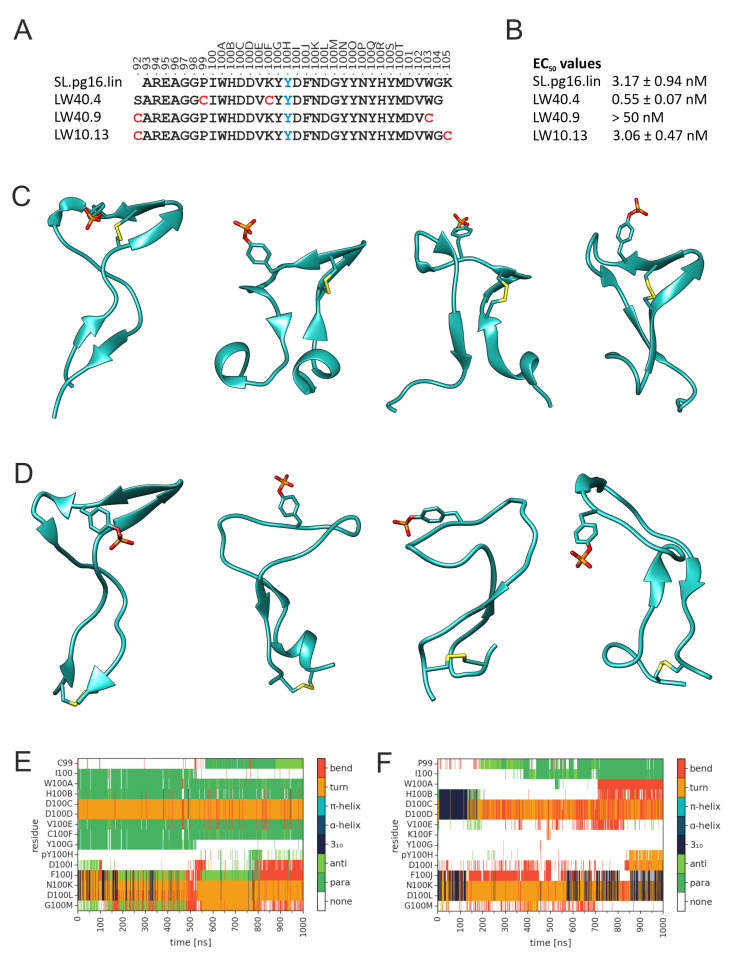
Figure 8.
Effect of disulfide bonds on the stability of PG16-CDRH3-derived peptides. (A) Schematic presentation of the alternative disulfide bonding patterns investigated. Cysteines forming an intramolecular disulfide bond are highlighted in red. Position 100H highlighted in blue represents a phosphotyrosine. (B) Experimental EC50 values for binding of HIV-1 gp120 (HxBc2) to the disulfide-bonded peptides in comparison to the linear peptide SL.pg16.lin. (C) Snapshots from the MD simulation illustrating the dynamics of the free LW40.04 peptide. The initial structure is shown in the left panel. The position of the disulfide bond and of the phosphotyrosine is indicated as sticks. (D) Snapshots from the MD simulation illustrating the dynamics of the free LW40.9 peptide. The initial structure is shown in the left panel. The position of the disulfide bond and of the phosphotyrosine is indicated as sticks. (E,F) Conformational stability of the central -hairpin in (E) LW40.4 and (F) LW40.9. The color code for the different types of secondary structure is given in the bar on the right.