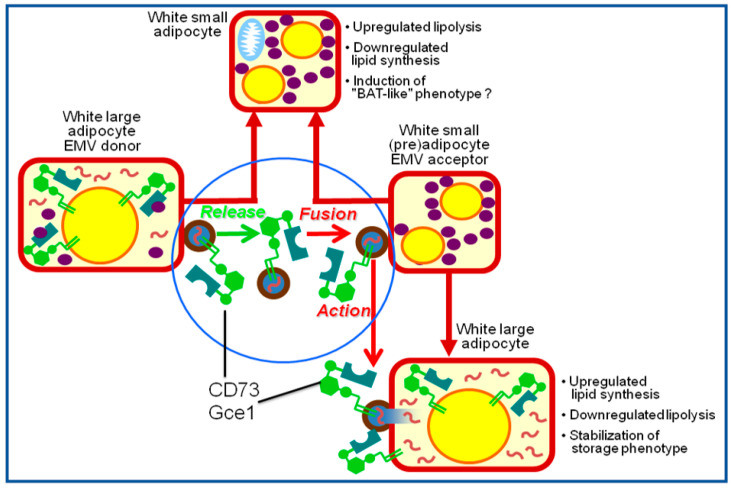
Figure 1.
Model for the involvement of interadipocyte material transfer with regard to the GPI-APs Gce1 and CD73 for the regulation of lipid metabolism, storage, and cell size within adipose tissue depots. The model is based on a polarized structure of adipose tissue depots with large mature adipocytes harboring a single or a few LD(s) only. These are capable of very pronounced esterification in parallel with high lipolysis and located in the immediate vicinity of blood vessels, where they form a gradient together with nascent adipocytes of small and very small size. Prolonged delivery of free fatty acids by the blood vessels and their resulting efficient esterification into LDs will cause the size increase of the adipocytes and exhaustion of their lipid storage capability. This will lead to the upregulation of the lipolytic release of fatty acids from their LDs into the interstitial space. This will only trigger the maturation of (very) small precursor adipocytes lacking visible LDs in the deeper adipose tissue layers that are capable of esterification and lipolysis to a very moderate degree by one or several types of intercellular signaling. Upon induction of novel and specific cell-to-cell contacts, as are already formed between the large adipocytes, or the (vesicular) secretion of adipokines [67] such as acylation stimulation protein ASP [68], or soluble factors such as 15-keto-PGE2 [69,70], the targeted cell adhesion molecules or adipokine receptors at the cell surface or nuclear hormone receptors, respectively, induce the signaling for stimulation of the esterification and/or inhibition of lipolysis. This in concert finally leads to upregulated LD biogenesis in the growing small adipocytes. Alternatively, or in addition, upon being challenged with high concentrations of fatty acids originating directly from the blood vessels or from lipolytic release in response to physiological or pharmacological stimuli such as hydrogen peroxide or the anti-diabetic sulfonylurea drug (SU) glimepiride [71], the large adipocytes release Gce1 and CD73 into EVs. Following passage through interstitial tissue spaces and eventually thereafter through the circulation, the EVs interact with the PMs of (very) small adipocytes for subsequent transfer of Gce1 and CD73 to the surface of LDs. Upon arrival, these enzymes degrade (c)AMP. Again, the resulting parallel upregulation of esterification and downregulation of lipolysis lead to the enlargement of the LDs and size of the adipocytes.