Abstract
Simple Summary
Cutaneous side effects are among the most frequently reported adverse reactions of modern dermato-oncological therapies, such as immune checkpoint inhibitors and targeted therapies. This study aims to provide a detailed overview of the cutaneous toxicity profile of these treatments to facilitate physicians’ early recognition of these side effects. Furthermore, we aim to accentuate the need for a dermatological evaluation of the affected patients, as it can significantly affect the patient’s quality of life and the decision to continue treatment.
Abstract
The advent of immunotherapy and targeted therapies in treating dermatological malignancies has dramatically changed the landscape of dermato-oncology in recent years. Their superior efficacy compared to previous therapeutic options, such as chemotherapy, has resulted in their use in treating devastating malignancies, such as melanoma or unresectable/metastatic basal cell and squamous cell carcinoma. Skin toxicity is a critical safety consideration, among other adverse reactions, that can occur under treatment with these agents. This article aims to summarize the cutaneous side effects of immune checkpoint inhibitors and targeted dermato-oncological therapies. Although the skin side effects of these agents are primarily mild, they can occasionally affect the decision for treatment continuation and the quality of life of the affected patients. Therefore, physicians must be acquainted with the specific cutaneous toxicity profile of such treatments to mitigate their impact on the patients and optimize the overall outcome of dermato-oncological therapy.
Keywords: immune checkpoint inhibitors, targeted therapy, cutaneous side effects, melanoma, basal cell carcinoma, squamous cell carcinoma
1. Introduction
Immune checkpoint-inhibitors (ICIs) and targeted therapies, such as BRAF-inhibitors (BRAFIs) or MEK-inhibitors (MEKis), have revolutionized the management of patients with melanocytic dermatological malignancies, such as melanoma [1,2]. Furthermore, significant progress has been achieved in the treatment of unresectable Nonmelanoma Skin Cancer (NMSC), such as advanced basal cell carcinomas and cutaneous squamous cell carcinomas, with the introduction of both Hedgehog inhibitors and ICIs, respectively, in the armamentarium of dermato-oncologists [3,4].
ICIs are antibodies targeting checkpoint proteins, such as T lymphocyte-associated antigen-4 (CTLA-4), programmed cell death protein 1 (PD-1), or programmed death ligand 1 (PD-L1) [2,5]. These proteins are crucial for maintaining the immunological equilibrium: CTLA-4 regulates the early T-cell-associated immunologic activation, while PD-1 and PD-L1 help to regulate the late T-cell-activation in the peripheral tissues [5,6]. Furthermore, checkpoint proteins attenuate the amplitude of the immune activation and therefore allow tolerance towards the circulation of cancer cells [2,5]. Thus, the immunologic activation via immune checkpoint inhibition can result in enhanced anti-tumor activity [5,7]. Nonetheless, this mechanism of ICIs that produces such remarkable therapeutic results can also cause immune-mediated adverse reactions that often involve the skin [8].
When it comes to targeted therapies, the combination of BRAFis and MEKis has also significantly prolonged the overall survival of melanoma patients via regulation of the mitogen-activated protein kinase (MAPK) pathway [9,10]. As this pathway plays a significant role in regulating cell proliferation and differentiation, mutations in the involved genes are crucial in the development of melanoma [11]. Activating BRAF mutations are present in approximately 40–60% of patients with cutaneous melanomas, making the latter eligible for treatment with agents such as vemurafenib or dabrafenib [10]. The introduction of BRAFis was followed by the approval of MEKis, also potent inhibitors of the MAPK signaling pathway [10,11]. The combined BRAFi/MEKi therapy has been established as a first-line melanoma treatment since then, and it has demonstrated excellent therapeutic outcomes and relatively good tolerability; however, side effects have also been frequently documented, primarily due to reactivation of the MAPK signaling pathway [12,13]. It is now well established that adding a MEK inhibitor to a BRAF inhibitor has superior results in terms of survival compared to BRAF monotherapy [14]. Interestingly, the combined BRAFi/MEKi therapy seems to be less associated with skin toxicity than BRAF monotherapy [15]. Nevertheless, cutaneous adverse reactions with these agents are relatively common; therefore, physicians should be well informed concerning their management [12,16].
Finally, vismodegib and sonidegib are effective smoothened-homologue (SMO) inhibitors that have shown excellent efficacy in the treatment of advanced, non-resectable basal cell carcinomas (BCC) [3]. BCCs are characterized by an over-activated hedgehog signaling pathway due to mutations in the patched homologue 1 (PTCH1) [3]. These mutations result in uncontrolled SMO activation, thus leading to increased cell proliferation and survival [3]. Hedgehog inhibitors block this over-activated signaling pathway and prevent tumor progression [3]. However, this type of treatment is often associated with adverse events that may lead to drug discontinuation [17]. In addition, mucocutaneous side effects of Hedgehog inhibitors are very common, and their correct management is crucial for better patient tolerance [17].
This review aims to provide a summarized overview of the skin toxicities caused by ICIs and targeted tumor therapies to assist physicians in their prompt recognition. The detailed management of such manifestations is beyond the scope of this review; therefore, it will not be further elaborated.
2. Materials and Methods
The present study is a narrative review. Literature research was conducted by querying the following databases: MEDLINE (PubMed) and SCOPUS. The Mesh key terms that were used included the following: “ICIs” or “BRAF-inhibitor”, or “MEK-inhibitor” or” ipilimumab” or “pembrolizumab” or “nivolumab” or “relatlimab” or “cemiplimab” or “vemurafenib” or “dabrafenib” or “bimetinib” or “encorafenib” or” cobimetinib” or “trametinib” or “vismodegib” or “sonidegib” AND “skin toxicity” or “cutaneous adverse reactions” or “cutaneous side effects”. The research included articles published in English and German during the period 2010–2023. The reference lists of the papers retrieved during this process were also scanned for eligibility. Our search includes only articles that were published in the English and German languages. The selection process was performed in three steps: firstly, scanning of article titles and abstracts; secondly, exclusion of the non-relevant literature; and finally, evaluation of the remaining full-text papers (Figure 1).
Figure 1.
Review Flowchart.
3. Results
3.1. ICIs
3.1.1. Maculopapular Rash
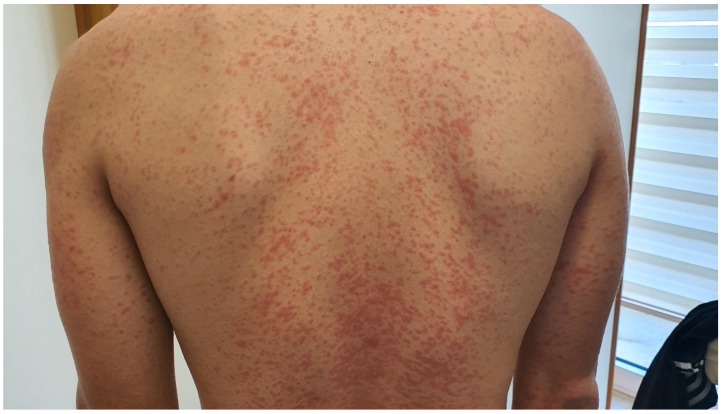
The maculopapular rash is the most frequently reported cutaneous irAE (immune-related adverse event) of PD-1/PD-L1 and CTLA-4 inhibitors [2]. It is most commonly seen with CTLA-4 inhibitors or under dual anti-PD1/PD-L1 and anti-CTLA therapy [18,19,20]. Although its severity can vary, grade ≥3 skin rashes, which are defined by the involvement of more than 30% of BSA (body surface area), are not frequent (Table 1) [2,5]. The rash appears as a nonspecific morbilliform or maculopapular exanthem that mainly involves the trunk, with a tendency to affect the extremities but sparing the face [21,22] (Figure 2). A prevalence of photo-exposed body areas has been documented, although that is not always the case [23]. The lesions can be asymptomatic or mildly pruritic [24]. Pruritus can either appear concomitantly with the skin reaction or precede the appearance of the lesions [2]. The outbreak can be documented as early as a few days after the administration of the first cycle or even in a delayed manner, appearing as late as several months after treatment initiation [5,25].
Table 1.
Skin toxicity grading [26].
| Adverse Reaction | Grace 1 | Grade 2 | Grade 3 | Life-Threatening Reactions |
|---|---|---|---|---|
| Maculopapular rash | Macules/papules covering\10% BSA with or without symptoms (e.g., pruritus, burning, tightness) |
Macules/papules covering 10–30% BSA with or without symptoms (e.g., pruritus, burning, tightness) | Macules/papules covering 30% BSA with or without associated symptoms; limiting self-care activities | - |
| Pruritus | Mild or localized; topical intervention indicated | Intense or widespread; intermittent; skin changes from scratching (e.g., edema, papulation, excoriations, lichenification, oozing/crusts); oral intervention indicated | Intense or widespread; constant; limiting self-care activities or sleep; oral corticosteroid or immunosuppressive therapy indicated |
Figure 2.
Maculopapular rash associated with Pembrolizumab in a patient with melanoma.
This rash’s most frequent histopathological characteristics include spongiotic dermatitis with associated superficial perivascular T-lymphocyte infiltrate, mimicking a cutaneous hypersensitivity reaction. In contrast, a lichenoid reaction can be documented less commonly [25,27]. Regarding other diagnostic criteria, peripheral eosinophilia concomitant with the rash appearance has been reported for both PD-1/PD-L1 and CTLA-4 inhibitors [5,24].
As this nonspecific rash can also represent an early manifestation of other more specific irAEs, such as psoriasis or bullous eruptions, a dermatological evaluation is necessary for specific classification and correct diagnosis [28]. This type of irAE is quite frequent, and, as it is benign and often self-limiting, it usually does not require aggressive management [5].
3.1.2. Lichenoid Rash
Lichenoid cutaneous drug reactions constitute another class of frequently encountered skin adverse events, and some experts believe they are almost as frequent as the nonspecific maculopapular rash [2,5,25]. This type of skin eruption tends to appear later than maculopapular irAEs, several months after ICI initiation [2,29].
In terms of clinical presentation, lichenoid irAEs can appear as classical lichen planus lesions, with pruritic polygonal papules exhibiting Wickham striae, or in a papulosquamous and bullous form, with a predilection for the trunk and extremities [5,30,31]. Mucosal, inverse, or even palmoplantar distribution has also been documented, although not very frequently [23,32,33]. The intensity of pruritus can vary from mild to severe, significantly affecting the patient’s quality of life and subsequent therapeutic management [2].
Diagnosis requires a histopathological examination, with which signs of interface dermatitis with a band-like lymphocytic infiltrate along the dermo-epidermal junction, vacuolization, and apoptotic keratinocytes can be documented [25,31,33]. In addition, hypergranulosis, acanthosis, spongiosis, and occasionally parakeratosis have also been reported [25,34]. Dermoscopic criteria that can further facilitate diagnosis include the presence of Wickham striae on the lesions [2].
3.1.3. Psoriasis or Psoriasiform Rash
Treatment with both PD-1/PD-L1 and CTLA-4 inhibitors entails a risk for the development of psoriasis or exacerbation of a pre-existing psoriatic disease [35,36]. Neither the exact incidence nor the detailed pathogenetic mechanism of this side effect has been completely elucidated [5]. Some suggest that this is a result of reactive overexpression of proinflammatory cytokines of the Th-17 signaling pathway as a response to the inhibition of PD-1 [37]. PD-1 normally downregulates the Th17 axis; therefore, its inhibition can generate a cascade that results in an immune-mediated T-cell activation with a shift towards a cytotoxic CD4+/CD8+ immunological status [38].
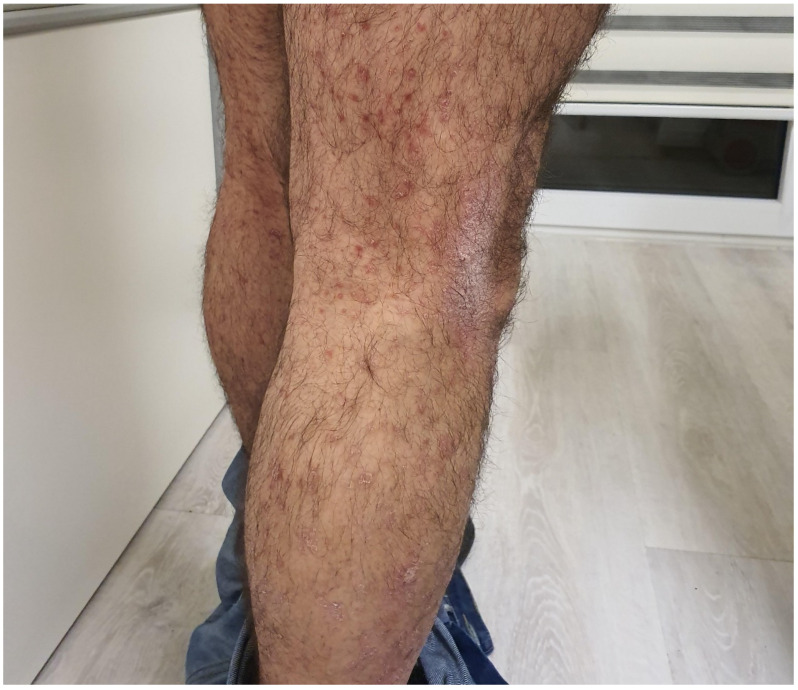
The clinical presentation of ICI-mediated psoriasis involves mostly the appearance of typical erythematosquamous plaques, while pustular, inverse, and guttate phenotypes have also been described [39,40] (Figure 3). Palmoplantar and scalp psoriasis, as well as the worsening of pre-existing or the appearance of newly acquired psoriatic arthritis, are also reported [2,39,40]. Histological findings do not differ from those of typical psoriasis vulgaris [41].
Figure 3.
Psoriasiform lesions associated with ipilimumab in a patient with melanoma.
3.1.4. Bullous Eruptions
The appearance of autoimmune bullous disorders under treatment with PD-1/PD-L1 and CTLA-4 inhibitors has been well demonstrated, with most reports indicating a higher prevalence under PD-1/PD-L1 inhibitors compared to anti-CTLA-4 inhibitors [2,42]. Prevalence is estimated to range between 1 and 8% of patients receiving ICIs, and the majority of cases are reported to occur with PD-1/PD-L1 inhibitors [43,44,45]. Exacerbation of pre-existing bullous pemphigoid has been mostly reported under anti-CTLA-4 agents [46].
Most cases of bullous irAEs involve bullous pemphigoid, with a disease onset varying from a few weeks to several months following treatment initiation [47]. Prior to the appearance of blisters, most patients notice pruritus alone or combined with erythematous and/or urticarial rashes, while mucosal involvement is rather uncommon [47,48,49]. Emerging reports on the appearance of newly acquired pemphigus vulgaris or exacerbation of pre-existing pemphigus disease under both PD-1/PD-L1 and CTLA-4 inhibitors are also present in the literature [50,51,52]. Cases of paraneoplastic pemphigus have also been reported and are associated mostly with the administration of PD-1/PD-L1 inhibitors [53,54]. Isolated cases of bullous lichenoid dermatitis, dermatitis herpetiformis, and linear IgA bullous dermatosis are also documented [2,5,27].
Diagnosis requires confirmation via histopathologic evaluation, immunohistochemistry, as well as findings from direct and indirect immunofluorescence [2]. The pathogenetic mechanism is not fully understood, but it is believed to be a result of excessive T-cell activation against bullous-pemphigoid-associated antigens that can also be expressed on the surface of tumor cells [49,55].
3.1.5. Pruritus
Pruritus is among the most common cutaneous adverse reactions under ICIs [42]. The prevalence of this complication is higher under monotherapy with ipilimumab or combination therapy of ipilimumab/nivolumab, as opposed to anti-PD-L1 treatment [5,56,57,58]. It can present either alone, in combination with nonspecific maculopapular rashes, or due to other conditions such as xerosis and other ICIs-associated dermatoses, including psoriasis [20,59]. Despite its benign nature, pruritus of intense severity can significantly affect the life quality of patients, and, therefore, it should be addressed accordingly (Table 1) [2].
3.1.6. Vitiligo
Vitiligo is another relatively frequent irAE, with an incidence of approximately 11% and 25% with CTLA-4 & PD-1/PDL1 inhibitors, respectively [5,14,56]. ICIs-related hypopigmentation is reported to occur as frequently as 2–8% in melanoma patients; however, its prevalence in patients with other malignities is unknown [42]. It is only sporadically mentioned as a treatment-related complication in various malignancies, including non-small cell lung cancer or soft-tissue sarcoma [60,61,62,63].
Regarding pathogenesis, a cytotoxic action of CD8+ activated T-cells against healthy melanocytes that share specific antigens with tumor melanocytes, such as Melan-A or gp100, is speculated [64,65]. The onset of this adverse reaction is usually several months after treatment initiation, and it is not a dose-related phenomenon [66].
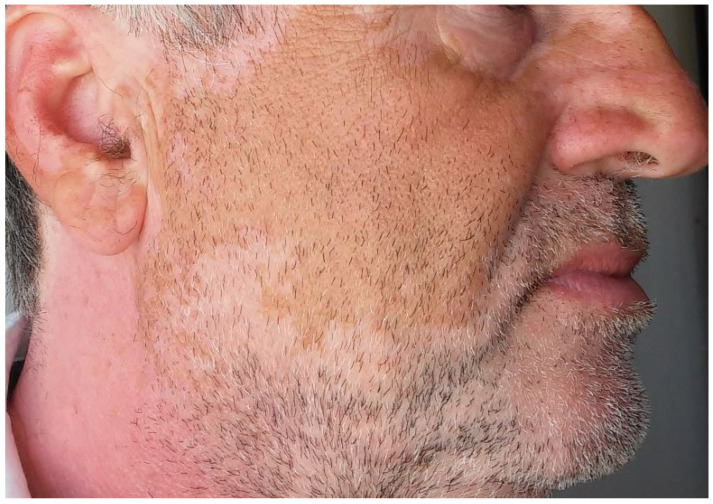
Clinically, lesions can be either focal and/or localized or generalized with a symmetrical distribution (Figure 4 [65]. Hair depigmentation, including eyebrows and eyelashes, can occur, while reports on regression of pre-existing pigmented lesions, such as nevi and solar lentigines, are also present in the literature [23,67]. Interestingly, a case series reporting hair repigmentation in 14 patients treated with PD-1/PD-L1 inhibitors for lung cancer is also present in the literature [68,69]. Although vitiligo does not seem to resolve after treatment discontinuation, its occurrence is frequently associated with a favorable therapeutic outcome and prolonged overall survival [70,71,72].
Figure 4.
Vitiligo under pembrolizumab in a patient with melanoma.
3.1.7. Hair and Nail Toxicity
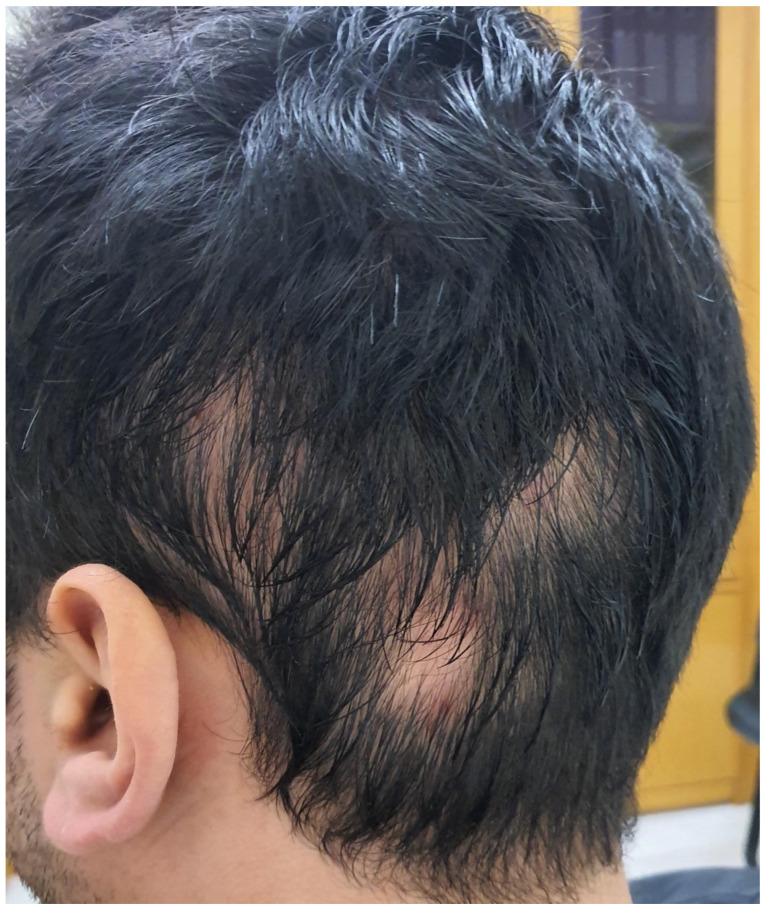
The most frequently encountered hair disorder among melanoma patients that receive ICIs is alopecia areata, which can be either focal or diffuse [23,73,74] (Figure 5). It tends to be more severe under CTLA-4 inhibitors than PD-1/PD-L1 inhibitors [5]. The pathogenic mechanism behind this irAE is believed to be a CD4+ and CD8+ T cell-mediated immune reaction triggered by PD-L1 that is present in cells of the hair follicle sheath [75].
Figure 5.
Alopecia areata under pembrolizumab in a patient with melanoma.
The onset of ICI-related alopecia areata is usually 3–6 months after treatment initiation [73]. In the case of hair regrowth in the affected areas, the newly grown hair could show signs of poliosis or a difference in hair structure [73,76]. As mentioned above, isolated cases of hair repigmentation under PD-1/PD-L1 treatment have also been documented [69]. Regarding other hair toxicities, besides alopecia areata, cases of telogen effluvium have also been reported [42].
Regarding nail toxicity, most of the isolated reported A.E.s to describe nonspecific onychodystrophia, onychomadesis, onycholysis, and paronychia [5,75].
3.1.8. Mucosal Toxicity
Inflammation of the oral mucosa manifesting as nonspecific stomatitis, periodontitis, and lichenoid reaction is relatively common and, in fact, better documented under treatment with PD-1/PD-L1 inhibitors, as opposed to CTLA-4 inhibitors [34,77,78].
Dysgeusia and xerostomia of diverse severity have also been documented, especially under anti-PD-1/PD-L1 treatment, and they are attributed to CD4/CD8-T-cell toxicity against the salivary glands [5,79]. Periodontitis is also believed to occur due to a T-cell-mediated inflammation that could be so severe as to provoke even tooth loss due to alveolar bone absorption [77].
Lichenoid reactions usually exhibit Wickham striae over an erythematous plaque or appear as papules, ulcers, and atrophic patches [42,80]. Lesions can also affect genital and perianal areas and can be either asymptomatic or mildly painful and sore [42]. ICI-associated mucosal toxicity might not be life-threatening; however, it can significantly interfere with the patients’ quality of life, and prompt management is necessary to avoid facing treatment modification dilemmas [75,81,82].
3.1.9. Potentially Life-Threatening Adverse Reactions
Severe or even potentially life-threatening cutaneous drug reactions, such as Stevens–Johnson syndrome, toxic epidermal necrolysis, acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms (DRESS), have been scarcely reported to occur under treatment with ICIs [81,83,84,85,86,87,88,89,90].
Both PD-1/PD-L1 inhibitors as well as CTLA-4 inhibitors can induce the appearance of these serious conditions [91,92]. Furthermore, these adverse reactions can occur even after a prolonged period of time from treatment initiation [86,87]. Finally, as nonspecific maculopapular rashes can precede the appearance of such potentially life-threatening eruptions, a careful dermatologic evaluation and, eventually, a histologic examination of a skin biopsy may be necessary to avoid devastating complications [8,93].
3.1.10. Other Cutaneous Adverse Reactions
Other ICI-associated cutaneous adverse reactions reported as case series or case reports are summarized alphabetically in Table 2.
Table 2.
Less frequently reported ICI-associated cutaneous adverse reactions.
| Less Frequently Encountered ICI-Associated irAEs |
|---|
| Acneiform rash/ papulopustular folliculitis [5,43,94] |
| Dermatomyositis [95,96,97] |
| Eruptive keratoacanthomas [98] |
| Erythema nodosum [99,100,101] |
| Grover’s disease [102,103,104] |
| Photosensitivity [5] |
| Pyoderma gangrenosum [105,106] |
| Sarcoidosis [107,108,109,110,111] |
| Sjogren’s syndrome [112] |
| Sweet syndrome [113,114] |
| Rosacea [115] |
| Urticaria [5] |
| Vasculitis [116,117] |
4. BRAF-Inhibitors
4.1. Photosensitivity
One of the most common side effects of BRAFis is photosensitivity, with an incidence ranging between 22.2% and 66.7% [9,118]. Most cases are related to vemurafenib compared to dabrafenib, and the mechanism of action is believed to be UVA-dependent photosensitivity [119,120].
The onset can be as early as a few days after treatment initiation, and it is mostly mild, although isolated more severe cases have also been documented [12,120,121]. Symptoms tend to intensify during the summer period, and the associated skin eruptions are more prevalent in sun-exposed body areas [120,121]. Facial or extra facial erythemas and actinic cheilitis of the lower lip are among the most frequent photosensitivity-associated documented skin adverse events [120]. Other symptoms include painful sunburns with or without blistering, with subsequent limitations for outdoor activities for the affected individuals [14].
4.2. NMSC (Nonmelanoma Skin Cancer) and Benign Cutaneous Neoplasias
The appearance of benign verrucous lesions is another frequently encountered adverse skin reaction in patients receiving BRAFis [122]. The role of HPV colonization in the pathogenesis of this side effect is controversial [14,122,123]. Verrucae vulgaris can appear on both sun-exposed and non-sun-exposed body areas, and they can appear as early as a few days after the initiation of BRAFi treatment; nonetheless, in the majority of cases, the onset is after four weeks of therapy [3,124]. Although they are benign, rare instances of malignant transformation to squamous cell carcinomas (SCC) have been reported [125]
Actinic keratoses (Aks) are also documented to occur frequently under BRAFi treatment, with an incidence among the studies ranging between 26 and 66.7% [118,123,124]. They occur mostly in sun-exposed areas, such as the scalp, face, and extremities, and they have a higher prevalence in photo-damaged individuals under BRAFis [14].
SCCs constitute the majority of NMSCs that can appear under therapy with BRAFis [9]. The mean onset time is estimated to be approximately seven weeks after treatment initiation, and they can appear de novo or on the ground of a pre-existing AK that has undergone malignant transformation [124]. Although the pathogenetic mechanism of this adverse reaction is not fully known, paradoxical activation of the MAPK signaling pathway in keratinocytes with pre-existing RAS mutations is speculated [12,126]. Similarly to AKs, SCCs also have a higher prevalence in sun-damaged skin, and they are mostly documented in patients over 49 years old [12,127]. Keratoacanthomas are well-differentiated SCCs and can also appear under BRAFi treatment [9].
4.3. Benign and Malignant Melanocytic Lesions
Therapy with BRAFis can induce changes in benign melanocytic nevi, such as changes of color and size or even involution of the lesions [128,129]. The mechanism behind nevi involution with BRAFis is believed to be the presence of the BRAF V600E mutation in the nevi melanocytes, which increases their susceptibility to treatment with the later agents [9]. In addition, eruptive melanocytic nevi with vemurafenib or encorafenib have also been described [130,131]. The explanation for this phenomenon, as well as for an increase in nevi size and pigmentation, is attributed to a BRAFi-assοciated proliferation of wild-type BRAF cells in the melanocytic lesions due to the activation of the RAS-RAF-MEK-ERK pathway, which induces increased cell proliferation and survival [130].
Lastly, the development of cutaneous melanomas de novo or arising from pre-existing nevi has also been described [128,132]. Newly developed melanomas can be either invasive or in situ, and the mean time of occurrence is estimated at approximately 4 to 12 weeks after treatment initiation [132,133].
4.4. Maculopapular Rash
Nonspecific maculopapular or morbilliform cutaneous eruptions are among the most frequent BRAFi-associated cutaneous adverse events [3,12]. The severity can range from grade 1 to grade 3; their incidence is documented to be between 64% and 75% in patients on BRAFi therapy, and the mean time of occurrence is approximately 1.6 weeks after treatment initiation [9,16]. Such eruptions can also occur in the face, trunk, and extremities [9]. Among the documented maculopapular or morbilliform eruptions are also keratosis-pilaris-like eruptions that are mostly asymptomatic, affecting arms and thighs. Notably, they are reported to occur more commonly under dabrafenib compared to vemurafenib [12,14].
4.5. Severe Cutaneous Adverse Reactions
Severe and potentially life-threatening cutaneous adverse events under treatment with BRAFis have also been documented. Such conditions include Stevens–Johnson syndrome, toxic epidermal necrolysis, DRESS, AGEP, and generalized bullous fixed eruption [10]. Most reported cases concern melanoma patients rather than other malignancies treated with BRAFis [10]. These types of adverse events are not frequent and tend to occur less commonly with dabrafenib–trametinib, encorafenib–binimetinib, and vemurafenib/cobimetinib, compared to vemurafenib monotherapy [134,135]. The mean time of onset is approximately 15.5 days for Steven’s–Johnson syndrome or toxic epidermal necrolysis and 11.4 days for DRESS, while a prior treatment with ICIs tends to increase the susceptibility to occurrence of a serious cutaneous adverse events [10].
4.6. Other Cutaneous Adverse Reactions
Other BRAFi-associated cutaneous adverse reactions, reported as case series or case reports, are summarized alphabetically in Table 3.
Table 3.
Less frequently reported BRAFi-associated cutaneous adverse events.
| Less Frequently Encountered BRAFi-Associated Cutaneous Adverse Events |
|---|
| Acneiform eruption [123,124] |
| Alopecia (telogen effluvium) [136] |
| Basal cell carcinoma [137] |
| Cheilitis [9] |
| Granulomatous eruption [138,139,140] |
| Grover’s disease [141] |
| Hand-foot skin reaction [142] |
| Milia [9] |
| Panniculitis [143,144] |
| Pruritus [10,14] |
| Vitiligo [145,146] |
| Xerosis [9] |
5. MEK-Inhibitors
5.1. Cutaneous Eruptions
The most frequently reported MEKi-associated cutaneous adverse event is a papulopustular eruption, with an incidence varying between 40% and 93% [147]. In terms of clinical presentation, this eruption tends to appear as pruritic papules and pustules in areas of high-sebum production, such as the scalp, face, and upper trunk [14]. In most documented cases, the severity of the papulopustular eruption is mild to moderate [148]. Such acneiform eruptions have been occasionally reported to co-exist with pruritus and erythema [148]. This type of skin toxicity is similar to the acneiform eruptions observed under epidermal growth factor receptor inhibitors (EGFRi), and it can occur as early as a few weeks under treatment initiation [14,149].
The mechanism of this adverse reaction is believed to be a MEKi-associated activation of the phosphoinositide 3-kinase (PI3K)-AKT pathway that subsequently results in an Insulin-like growing factor-1 (IGF-1)-mediated induction of sebaceous glands lipogenesis [150].
Other nonspecific cutaneous eruptions, such as maculopapular eruption with or without pruritus, have also been described under MEKis [148]. In addition, other clinical variations of maculopapular rashes have been sporadically described [151]. Patel et al. reported a case series of three patients who presented with a distinct drug hypersensitivity reaction on selumetinib, with disseminated lesions exhibiting an urticarial aspect and a characteristic central duskiness [151].
5.2. Other Cutaneous Findings
Other MEKi-associated dermatologic adverse reactions that are similar to EGRF-related skin toxicity include pruritus, xerosis, angular cheilitis, mucositis, paronychia, periungual fissuring, and hair disorders, such as alopecia, trichomegaly, and changes in hair texture and hair color [152]. The similarities between the skin toxicity profiles of MEKis and EGFR inhibitors are believed to derive from the direct inhibition of the MAPK pathway [153]. The safety profile of MEKis is favorable overall, causing milder cutaneous side effects compared to other systemic melanoma treatments [154].
Further MEKi-associated cutaneous adverse reactions, reported either as case series or case reports, are summarized alphabetically in Table 4.
Table 4.
Less frequently reported MEKi-associated cutaneous adverse events.
| Less Frequently Encountered MEKi-Associated Cutaneous Adverse Events |
|---|
| Angular cheilitis [14] |
| Cellulitis [153,155,156] |
| DRESS syndrome [153,155,156] |
| Hair disorders [152] |
| Mucositis [14] |
| Paronychia and periungual fissuring [14] |
| Psoriasiform scalp dermatitis |
| Pruritus [153] |
| Teleangiectasias [14] |
| Urticaria [153,155,156] |
| Xerosis [152] |
6. Hedgehog-Inhibitors
6.1. Alopecia
Alopecia is a well-documented cutaneous side effect of vismodegib, with a prevalence as high as 63% [157]. In addition, sonidegib-associated alopecia has also been documented [158]. The pathogenetic mechanism of this adverse reaction is based on the significant role of the Hedghog pathway in early hair follicle morphogenesis; therefore, inhibitory agents could induce an arrest in normal hair follicle development [157].
The onset of alopecia is usually approximately four months after treatment initiation [157]. The hair loss is gradual, and it can also affect body hair [159]. The severity of alopecia can vary from mild to ≥50% hair loss, and its clinical presentation can be either patchy or diffuse [159]. In most cases, alopecia resolves after therapy discontinuation, and the time frame for hair regrowth is approximately 6–12 months after treatment cessation [159,160]. However, even in the case of hair regrowth, the hair density could be permanently affected [159]. Isolated reports of alopecia persisting even longer than one year after treatment cessation have also been documented; it is, therefore, important to counsel patients accordingly [157].
6.2. Dysgeusia
Dysgeusia is another common side effect of treatment with hedgehog inhibitors, in approximately 51–85% of patients [161]. The pathogenic mechanism of this adverse reaction is believed to be a local effect of these agents in taste buds, resulting from the inhibition of the hedgehog-pathway signaling [162]. In a study by Yang et al., taste bud size and the number of taste cells per taste bud were significantly reduced in mice under vismodegib administration [162]. It was also demonstrated that the treatment with vismodegib led to a significant reduction of cells expressing molecules such as phospholipase Cβ2 or glucagon-like peptide-1, known modulators of sweet and bitter taste sensitivity [162].
The time of onset of dysgeusia or ageusia is about 1.3 to 6.5 months after treatment initiation, and clinical symptoms can vary [159]. Some patients experience a complete loss of taste, others experience a metallic taste, while others notice an increased taste of sweet or salty sensations [159]. Occurrences of unpleasant tastes or changes in taste in alcohol have also been documented [159]. Due to this adverse reaction, a large patient group often loses interest in food intake, which can lead to severe malnutrition and weight loss [159,161].
Further cutaneous adverse reactions under treatment with hedgehog inhibitors that have been less frequently reported are summarized alphabetically in Table 5.
Table 5.
Less frequently encountered cutaneous adverse reactions under treatment with hedgehog inhibitors.
| Less Frequently Encountered Cutaneous Adverse Reactions under Treatment with Hedgehog Inhibitors |
|---|
| AGEP [163] |
| Cutaneous eruptions (maculopapular, papulopustular) [164] |
| Folliculitis [160] |
| Grover’s disease [164] |
| Hypersensitivity reactions [160] |
| Keratoacanthomas [165] |
| Stevens-Johnson syndrome/Toxic epidermal necrolysis [163] |
7. Conclusions and Future Directions
With the increasing use of ICIs and targeted therapies in treating dermatologic malignancies, physicians gain a better understanding of their toxicity profile, both in terms of early recognition and adequate management [10]. Although the majority of cutaneous side effects of the aforementioned treatments are relatively mild, severe adverse reactions can also occur, affecting patient satisfaction and even treatment continuation [2]. It is, therefore, evident that multidisciplinary management of these cases, involving a dermatological assessment, is critical to ensure optimal patient treatment and a successful therapeutic outcome overall.
Abbreviations
| AGEP | Acute Generalized Exanthematous Pustulosis |
| BRAFi | BRAF-inhibitors |
| BSA | Body Surface Area |
| CTLA-4 | T lymphocyte-associated antigen-4 |
| DRESS | Drug Reaction with Eosinophilia and Systemic Symptoms |
| EGFR | Epidermal Growth Factor Receptor Inhibitors |
| ICIs | Immune Checkpoint-Inhibitors |
| IGF-1 | Insulin-like Growing Factor-1 |
| irAE | Immune-related Adverse Event |
| MAPK | Mitogen-activated Protein Kinase |
| MEKi | MEK-inhibitors |
| NMSC | Nonmelanoma Skin Cancer |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death Ligand 1 |
| PTCH1 | Patched Homologue 1 |
| SCC | Squamous Cell Carcinomas |
| SMO | Smoothened Homologue |
Author Contributions
Conceptualization, S.G. and K.-M.P.; Methodology, K.-M.P.; Software, K.-M.P.; Writing—Original Draft Preparation, K.-M.P., V.F. and V.G.; Writing—Review and Editing, S.G., V.F., V.G. and K.-M.P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chen C.-H., Yu H.-S., Yu S. Cutaneous Adverse Events Associated with Immune Checkpoint Inhibitors: A Review Article. Curr. Oncol. 2022;29:2871–2886. doi: 10.3390/curroncol29040234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apalla Z., Papageorgiou C., Lallas A., Delli F., Fotiadou C., Kemanetzi C., Lazaridou E. Cutaneous Adverse Events of Immune Checkpoint Inhibitors: A Literature Review. Dermatol. Pract. Concept. 2021;11:e2021155. doi: 10.5826/dpc.1101a155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macdonald J.B., Macdonald B., Golitz L.E., LoRusso P., Sekulic A. Cutaneous adverse effects of targeted therapies: Part II: Inhibitors of intracellular molecular signaling pathways. [(accessed on 11 March 2023)];J. Am. Acad. Dermatol. 2015 72:221–236. doi: 10.1016/j.jaad.2014.07.033. Available online: https://pubmed.ncbi.nlm.nih.gov/25592339. [DOI] [PubMed] [Google Scholar]
- 4.Yokota T., Homma A., Kiyota N., Tahara M., Hanai N., Asakage T., Matsuura K., Ogawa T., Saito Y., Sano D., et al. Immunotherapy for squamous cell carcinoma of the head and neck. Jpn. J. Clin. Oncol. 2020;50:1089–1096. doi: 10.1093/jjco/hyaa139. [DOI] [PubMed] [Google Scholar]
- 5.Sibaud V. Dermatologic Reactions to Immune Checkpoint Inhibitors: Skin Toxicities and Immunotherapy. [(accessed on 6 March 2023)];Am. J. Clin. Dermatol. 2018 19:345–361. doi: 10.1007/s40257-017-0336-3. Available online: https://pubmed.ncbi.nlm.nih.gov/29256113. [DOI] [PubMed] [Google Scholar]
- 6.Luke J.J., Ott P.A. PD-1 pathway inhibitors: The next generation of immunotherapy for advanced melanoma. [(accessed on 6 March 2023)];Oncotarget. 2015 6:3479–3492. doi: 10.18632/oncotarget.2980. Available online: https://pubmed.ncbi.nlm.nih.gov/25682878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pennock G.K., Chow L.Q.M. The Evolving Role of Immune Checkpoint Inhibitors in Cancer Treatment. [(accessed on 6 March 2023)];Oncologist. 2015 20:812–822. doi: 10.1634/theoncologist.2014-0422. Available online: https://pubmed.ncbi.nlm.nih.gov/26069281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muntyanu A., Netchiporouk E., Gerstein W., Gniadecki R., Litvinov I.V. Cutaneous Immune-Related Adverse Events (irAEs) to Immune Checkpoint Inhibitors: A Dermatology Perspective on Management. J. Cutan. Med. Surg. 2020;25:59–76. doi: 10.1177/1203475420943260. [DOI] [PubMed] [Google Scholar]
- 9.Gençler B., Gönül M. Cutaneous Side Effects of BRAF Inhibitors in Advanced Melanoma: Review of the Literature. Dermatol. Res. Pract. 2016;2016:1–6. doi: 10.1155/2016/5361569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torres-Navarro I., de Unamuno-Bustos B., Botella-Estrada R. Systematic review of BRAF/MEK inhibitors-induced Severe Cu-taneous Adverse Reactions (SCARs) J. Eur. Acad. Dermatol. Venereol. 2021;35:607–614. doi: 10.1111/jdv.16894. [DOI] [PubMed] [Google Scholar]
- 11.Naqash A.R., File D.M., Ziemer C.M., Whang Y.E., Landman P., Googe P.B., Collichio F.A. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. A single-institutional case-series. J. Immunother. Cancer. 2019;7:4. doi: 10.1186/s40425-018-0475-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lacouture M.E., Duvic M., Hauschild A., Prieto V.G., Robert C., Schadendorf D., Kim C.C., McCormack C.J., Myskowski P.L., Spleiss O., et al. Analysis of Dermatologic Events in Vemu-rafenib-Treated Patients with Melanoma. Oncologist. 2013;18:314–322. doi: 10.1634/theoncologist.2012-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hertzman Johansson C., Egyhazi Brage S. BRAF inhibitors in cancer therapy. Pharmacol. Ther. 2014;142:176–182. doi: 10.1016/j.pharmthera.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 14.De Golian E., Kwong B.Y., Swetter S.M., Pugliese S.B. Cutaneous Complications of Targeted Melanoma Therapy. [(accessed on 11 March 2023)];Curr. Treat. Options Oncol. 2016 17:57. doi: 10.1007/s11864-016-0434-0. Available online: https://pubmed.ncbi.nlm.nih.gov/27645330. [DOI] [PubMed] [Google Scholar]
- 15.Carlos G., Anforth R., Clements A., Menzies A.M., Carlino M.S., Chou S., Fernandez-Peñas P. Cutaneous Toxic Effects of BRAF Inhibitors Alone and in Combination with MEK Inhibitors for Metastatic Melanoma. [(accessed on 13 March 2023)];JAMA Dermatol. 2015 151:1103–1109. doi: 10.1001/jamadermatol.2015.1745. Available online: https://pubmed.ncbi.nlm.nih.gov/26200476. [DOI] [PubMed] [Google Scholar]
- 16.Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. [(accessed on 12 March 2023)];N. Engl. J. Med. 2011 364:2507–2516. doi: 10.1056/NEJMoa1103782. Available online: https://pubmed.ncbi.nlm.nih.gov/21639808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sekulic A., Migden M.R., Oro A.E., Dirix L., Lewis K.D., Hainsworth J.D., Solomon J.A., Yoo S., Arron S.T., Friedlander P.A., et al. Efficacy and Safety of Vismodegib in Advanced Basal-Cell Carcinoma. [(accessed on 15 March 2023)];N. Engl. J. Med. 2012 366:2171–2179. doi: 10.1056/NEJMoa1113713. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5278761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oniangue-Ndza C., Gooden K.M., May J., Malcolm B., Du E.X., Yin L., Betts K.A. PCN111 comparison of adverse event costs of nivolumab+ipilimumab versus sunitinib for previously untreated intermediate-/poor-risk ad-vanced renal cell carcinoma in Switzerland. Value Health. 2019;22:S457. doi: 10.1016/j.jval.2019.09.308. [DOI] [Google Scholar]
- 19.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P., et al. Combined Nivolumab and Ipilimumab or Mono-therapy in Untreated Melanoma. N. Engl. J. Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber J.S., Hodi F.S., Wolchok J.D., Topalian S.L., Schadendorf D., Larkin J., Sznol M., Long G.V., Li H., Waxman I.M., et al. Safety Profile of Nivolumab Monotherapy: A Pooled Analysis of Patients with Advanced Melanoma. J. Clin. Oncol. 2017;35:785–792. doi: 10.1200/JCO.2015.66.1389. [DOI] [PubMed] [Google Scholar]
- 21.Robert C., Long G.v., Brady B., Dutriaux C., Maio M., Mortier L., Hassel J.C., Rutkowski P., McNeil C., Kalinka-Warzocha E., et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 22.Tawbi H.A., Schadendorf D., Lipson E.J., Ascierto P.A., Matamala L., Gutiérrez E.C., Rutkowski P., Gogas H.J., Lao C.D., De Menezes J.J., et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. [(accessed on 17 May 2023)];N. Engl. J. Med. 2022 386:24–34. doi: 10.1056/NEJMoa2109970. Available online: https://pubmed.ncbi.nlm.nih.gov/34986285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann L., Forschner A., Loquai C., Goldinger S.M., Zimmer L., Ugurel S., Schmidgen M.I., Gutzmer R., Utikal J.S., Göppner D., et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer. 2016;60:190–209. doi: 10.1016/j.ejca.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Lacouture M.E., Wolchok J.D., Yosipovitch G., Kähler K.C., Busam K.J., Hauschild A. Ipilimumab in patients with cancer and the management of dermatologic adverse events. J. Am. Acad. Dermatol. 2014;71:161–169. doi: 10.1016/j.jaad.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 25.Shi V.J., Rodic N., Gettinger S., Leventhal J.S., Neckman J.P., Girardi M., Bosenberg M., Choi J.N. Clinical and histologic features of lichenoid mucocu-taneous eruptions due to anti-programmed cell death 1 and anti-programmed cell death ligand 1 immunotherapy. JAMA Dermatol. 2016;152:1128–1136. doi: 10.1001/jamadermatol.2016.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen A.P., Setser A., Anadkat M.J., Cotliar J., Olsen E.A., Garden B.C., Lacouture M.E. Grading dermatologic adverse events of cancer treatments: The Common Terminology Criteria for Adverse Events Version 4.0. [(accessed on 17 May 2023)];J. Am. Acad. Dermatol. 2012 67:1025–1039. doi: 10.1016/j.jaad.2012.02.010. Available online: https://pubmed.ncbi.nlm.nih.gov/22502948. [DOI] [PubMed] [Google Scholar]
- 27.Curry J.L., Tetzlaff M.T., Nagarajan P., Drucker C., Diab A., Hymes S.R., Duvic M., Hwu W.-J., Wargo J.A., Torres-Cabala C.A., et al. Diverse types of dermatologic toxicities from immune checkpoint blockade therapy. [(accessed on 7 March 2023)];J. Cutan. Pathol. 2016 44:158–176. doi: 10.1111/cup.12858. Available online: https://pubmed.ncbi.nlm.nih.gov/27859479. [DOI] [PubMed] [Google Scholar]
- 28.Naidoo J., Page D.B., Li B.T., Connell L.C., Schindler K., Lacouture M.E., Postow M.A., Wolchok J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015;26:2375–2391. doi: 10.1093/annonc/mdv383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomes N., Sibaud V., Azevedo F., Magina S. Cutaneous Toxicity of Immune Checkpoint Inhibitors: A Narrative Review. [(accessed on 7 March 2023)];Acta Med. Port. 2020 33:335–343. doi: 10.20344/amp.12424. Available online: https://pubmed.ncbi.nlm.nih.gov/32416756. [DOI] [PubMed] [Google Scholar]
- 30.Joseph R.W., Cappel M., Goedjen B., Gordon M., Kirsch B., Gilstrap C., Bagaria S., Jambusaria-Pahlajani A. Lichenoid Dermatitis in Three Patients with Metastatic Melanoma Treated with Anti–PD-1 Therapy. [(accessed on 7 March 2023)];Cancer Immunol. Res. 2015 3:18–22. doi: 10.1158/2326-6066.CIR-14-0134. Available online: https://pubmed.ncbi.nlm.nih.gov/25287118. [DOI] [PubMed] [Google Scholar]
- 31.Schaberg K.B., Novoa R.A., Wakelee H.A., Kim J., Cheung C., Srinivas S., Kwong B.Y. Immunohistochemical analysis of lichenoid reactions in patients treated with anti-PD-L1 and anti-PD-1 therapy. [(accessed on 7 March 2023)];J. Cutan. Pathol. 2016 43:339–346. doi: 10.1111/cup.12666. Available online: https://pubmed.ncbi.nlm.nih.gov/26762844. [DOI] [PubMed] [Google Scholar]
- 32.Guggina L.M., Yanes D.A., Choi J.N. Inverse lichenoid drug eruption associated with nivolumab. [(accessed on 7 March 2023)];JAAD Case Rep. 2016 3:7–9. doi: 10.1016/j.jdcr.2016.11.002. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5198731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tetzlaff M.T., Nagarajan P., Chon S., Huen A., Diab A., Omar P., Aung P.P., Torres-Cabala C.A., Mays S.R., Prieto V.G., et al. Lichenoid Dermatologic Toxicity From Immune Checkpoint Blockade Therapy: A Detailed Examination of the Clinicopathologic Features. [(accessed on 7 March 2023)];Am. J. Dermatopathol. 2017 39:121–129. doi: 10.1097/DAD.0000000000000688. Available online: https://pubmed.ncbi.nlm.nih.gov/28134729. [DOI] [PubMed] [Google Scholar]
- 34.Sibaud V., Eid C., Belum V.R., Combemale P., Barres B., Lamant L., Mourey L., Gomez-Roca C., Estilo C.L., Motzer R., et al. Oral Lichenoid Reactions associated with anti-PD-1/PD-L1 therapies: Clinicopathological findings. [(accessed on 7 March 2023)];J. Eur. Acad. Dermatol. Venereol. 2017 31:e464. doi: 10.1111/jdv.14284. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5645209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahil S.K., Capon F., Barker J.N. Update on psoriasis immunopathogenesis and targeted immunotherapy. [(accessed on 7 March 2023)];Semin. Immunopathol. 2015 38:11–27. doi: 10.1007/s00281-015-0539-8. Available online: https://pubmed.ncbi.nlm.nih.gov/26573299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chia P.L., John T. Severe Psoriasis Flare After Anti-Programmed Death Ligand 1 (PD-L1) Therapy for Metastatic Non–Small Cell Lung Cancer (NSCLC) [(accessed on 7 March 2023)];J. Immunother. 2016 39:202–204. doi: 10.1097/CJI.0000000000000121. Available online: https://pubmed.ncbi.nlm.nih.gov/27163740. [DOI] [PubMed] [Google Scholar]
- 37.Dulos J., Carven G.J., van Boxtel S.J., Evers S., Driessen-Engels L.J.A., Hobo W., Gorecka M.A., de Haan A.F.J., Mulders P., Punt C.J.A., et al. PD-1 Blockade Augments Th1 and Th17 and Suppresses Th2 Responses in Peripheral Blood from Patients with Prostate and Advanced Melanoma Cancer. [(accessed on 7 March 2023)];J. Immunother. 2012 35:169–178. doi: 10.1097/CJI.0b013e318247a4e7. Available online: https://pubmed.ncbi.nlm.nih.gov/22306905. [DOI] [PubMed] [Google Scholar]
- 38.Matsumura N., Ohtsuka M., Kikuchi N., Yamamoto T. Exacerbation of Psoriasis During Nivolumab Therapy for Metastatic Melanoma. [(accessed on 7 March 2023)];Acta Derm. -Venereol. 2016 96:259–260. doi: 10.2340/00015555-2212. Available online: https://pubmed.ncbi.nlm.nih.gov/26270860. [DOI] [PubMed] [Google Scholar]
- 39.Totonchy M.B., Ezaldein H.H., Ko C., Choi J. Inverse Psoriasiform Eruption During Pembrolizumab Therapy for Metastatic Melanoma. [(accessed on 7 March 2023)];JAMA Dermatol. 2016 152:590–592. doi: 10.1001/jamadermatol.2015.5210. Available online: https://pubmed.ncbi.nlm.nih.gov/26675815. [DOI] [PubMed] [Google Scholar]
- 40.Bonigen J., Raynaud-Donzel C., Hureaux J., Kramkimel N., Blom A., Jeudy G., Breton A.-L., Hubiche T., Bedane C., Legoupil D., et al. Anti-PD1-induced psoriasis: A study of 21 patients. [(accessed on 7 March 2023)];J. Eur. Acad. Dermatol. Venereol. 2016 31:e254–e257. doi: 10.1111/jdv.14011. Available online: https://pubmed.ncbi.nlm.nih.gov/27739129. [DOI] [PubMed] [Google Scholar]
- 41.Ruiz-Bañobre J., Abdulkader I., Anido U., León L., López-López R., García-González J. Development of de novo psoriasis during nivolumab therapy for metastatic renal cell carcinoma: Immunohistochemical analyses and clinical outcome. [(accessed on 7 March 2023)];Apmis. 2017 125:259–263. doi: 10.1111/apm.12658. Available online: https://pubmed.ncbi.nlm.nih.gov/28233446. [DOI] [PubMed] [Google Scholar]
- 42.Geisler A.N., Phillips G.S., Barrios D.M., Wu J., Leung D.Y.M., Moy A.P., Kern J.A., Lacouture M.E. Immune checkpoint inhibitor–related dermatologic adverse events. [(accessed on 7 March 2023)];J. Am. Acad. Dermatol. 2020 83:1255–1268. doi: 10.1016/j.jaad.2020.03.132. Available online: https://pubmed.ncbi.nlm.nih.gov/32454097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang S.J.E., Carlos G., Wakade D., Byth K., Kong B.Y., Chou S., Carlino M.S., Kefford R., Fernandez-Penas P. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: A single-institution cohort. [(accessed on 7 March 2023)];J. Am. Acad. Dermatol. 2016 74:455–461.e1. doi: 10.1016/j.jaad.2015.10.029. Available online: https://pubmed.ncbi.nlm.nih.gov/26793994. [DOI] [PubMed] [Google Scholar]
- 44.Coleman E., Ko C., Dai F., Tomayko M.M., Kluger H., Leventhal J.S. Inflammatory eruptions associated with immune checkpoint inhibitor therapy: A single-institution retrospective analysis with stratification of reactions by toxicity and implications for management. [(accessed on 7 March 2023)];J. Am. Acad. Dermatol. 2019 80:990–997. doi: 10.1016/j.jaad.2018.10.062. Available online: https://pubmed.ncbi.nlm.nih.gov/30399387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siegel J., Totonchy M., Damsky W., Berk-Krauss J., Castiglione F., Sznol M., Petrylak D.P., Fischbach N., Goldberg S.B., Decker R.H., et al. Bullous disorders associated with anti-PD-1 and anti-PD-L1 therapy: A retrospective analysis evaluating the clinical and histopathologic features, frequency, and impact on cancer therapy. [(accessed on 7 March 2023)];J. Am. Acad. Dermatol. 2018 79:1081–1088. doi: 10.1016/j.jaad.2018.07.008. Available online: https://pubmed.ncbi.nlm.nih.gov/30025829. [DOI] [PubMed] [Google Scholar]
- 46.Beck K.M., Dong J., Geskin L.J., Beltrani V.P., Phelps R.G., Carvajal R.D., Schwartz G., Saenger Y.M., Gartrell R.D. Disease stabilization with pembrolizumab for metastatic acral melanoma in the setting of autoimmune bullous pemphigoid. [(accessed on 7 March 2023)];J. Immunother. Cancer. 2016 4:20. doi: 10.1186/s40425-016-0123-3. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4835882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Damsky W., Kole L., Tomayko M.M. Development of bullous pemphigoid during nivolumab therapy. [(accessed on 7 March 2023)];JAAD Case Rep. 2016 2:442–444. doi: 10.1016/j.jdcr.2016.05.009. Available online: https://pubmed.ncbi.nlm.nih.gov/27981213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sowerby L., Dewan A.K., Granter S., Gandhi L., Leboeuf N.R. Rituximab Treatment of Nivolumab-Induced Bullous Pemphigoid. [(accessed on 7 March 2023)];JAMA Dermatol. 2017 153:603–605. doi: 10.1001/jamadermatol.2017.0091. Available online: https://pubmed.ncbi.nlm.nih.gov/28355425. [DOI] [PubMed] [Google Scholar]
- 49.Naidoo J., Schindler K., Querfeld C., Busam K., Cunningham J., Page D.B., Postow M.A., Weinstein A., Lucas A.S., Ciccolini K.T., et al. Autoimmune Bullous Skin Disorders with Immune Checkpoint Inhibitors Targeting PD-1 and PD-L1. [(accessed on 7 March 2023)];Cancer Immunol. Res. 2016 4:383–389. doi: 10.1158/2326-6066.CIR-15-0123. Available online: https://pubmed.ncbi.nlm.nih.gov/26928461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garje R., Chau J.J., Chung J., Wanat K., Zakharia Y. Acute Flare of Bullous Pemphigus with Pembrolizumab Used for Treatment of Metastatic Urothelial Cancer. [(accessed on 7 March 2023)];J. Immunother. 2018 41:42–44. doi: 10.1097/CJI.0000000000000191. Available online: https://pubmed.ncbi.nlm.nih.gov/29111983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clawson R.C., Tabata M.M., Chen S.T. Pemphigus vulgaris flare in a patient treated with nivolumab. [(accessed on 7 March 2023)];Dermatol. Ther. 2021 34:e14871. doi: 10.1111/dth.14871. Available online: https://pubmed.ncbi.nlm.nih.gov/33571394. [DOI] [PubMed] [Google Scholar]
- 52.Krammer S., Krammer C., Salzer S., Bağci I.S., French L.E., Hartmann D. Recurrence of Pemphigus Vulgaris Under Nivolumab Therapy. [(accessed on 7 March 2023)];Front. Med. 2019 6:262. doi: 10.3389/fmed.2019.00262. Available online: https://pubmed.ncbi.nlm.nih.gov/31781569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yatim A., Bohelay G., Grootenboer-Mignot S., Prost-Squarcioni C., Alexandre M., Le Roux-Villet C., Martin A., Maubec E., Caux F. Paraneoplastic Pemphigus Revealed by Anti-programmed Death-1 Pembrolizumab Therapy for Cutaneous Squamous Cell Carcinoma Complicating Hidradenitis Suppurativa. [(accessed on 7 March 2023)];Front. Med. 2019 6:249. doi: 10.3389/fmed.2019.00249. Available online: https://pubmed.ncbi.nlm.nih.gov/31750309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McNally M.A., Vangipuram R., Campbell M.T., Nagarajan P., Patel A.B., Curry J.L., Heberton M. Paraneoplastic pemphigus manifesting in a patient treated with pembrolizumab for urothelial carcinoma. [(accessed on 7 March 2023)];JAAD Case Rep. 2021 10:82–84. doi: 10.1016/j.jdcr.2021.02.012. Available online: https://pubmed.ncbi.nlm.nih.gov/33778141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwon C., Land A., Smoller B., Scott G., Beck L., Mercurio M. Bullous pemphigoid associated with nivolumab, a programmed cell death 1 protein inhibitor. [(accessed on 7 March 2023)];J. Eur. Acad. Dermatol. Venereol. 2017 31:e349–e350. doi: 10.1111/jdv.14143. Available online: https://pubmed.ncbi.nlm.nih.gov/28129461. [DOI] [PubMed] [Google Scholar]
- 56.Sibaud V., Meyer N., Lamant L., Vigarios E., Mazieres J., Delord J.P. Dermatologic complications of anti-PD-1/PD-L1 immune checkpoint antibodies. [(accessed on 7 March 2023)];Curr. Opin. Oncol. 2016 28:254–263. doi: 10.1097/CCO.0000000000000290. Available online: https://pubmed.ncbi.nlm.nih.gov/27136138. [DOI] [PubMed] [Google Scholar]
- 57.Balar A.V., Galsky M.D., Rosenberg J.E., Powles T., Petrylak D.P., Bellmunt J., Loriot Y., Necchi A., Hoffman-Censits J., Perez-Gracia J.L., et al. Atezolizumab as first-line treatment in cispla-tin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. [(accessed on 7 March 2023)];Lancet. 2017 389:67–76. doi: 10.1016/S0140-6736(16)32455-2. Available online: https://pubmed.ncbi.nlm.nih.gov/27939400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peters S., Gettinger S., Johnson M.L., Jänne P.A., Garassino M.C., Christoph D., Toh C.K., Rizvi N.A., Chaft J.E., Costa E.C., et al. Phase II Trial of Atezolizumab as First-Line or Subsequent Therapy for Patients with Programmed Death-Ligand 1–Selected Advanced Non–Small-Cell Lung Cancer (BIRCH) [(accessed on 7 March 2023)];J. Clin. Oncol. 2017 35:2781–2789. doi: 10.1200/JCO.2016.71.9476. Available online: https://pubmed.ncbi.nlm.nih.gov/28609226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sanlorenzo M., Vujic I., Daud A., Algazi A., Gubens M., Luna S.A., Lin K., Quaglino P., Rappersberger K., Ortiz-Urda S. Pembrolizumab cutaneous adverse events and their asso-ciation with disease progression. JAMA Dermatol. 2015;151:1206–1212. doi: 10.1001/jamadermatol.2015.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yin E.S., Totonchy M.B., Leventhal J.S. Nivolumab-associated vitiligo-like depigmentation in a patient with acute myeloid leukemia: A novel finding. JAAD Case Rep. 2017;3:90–92. doi: 10.1016/j.jdcr.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mathias C., Wakelee H.I. 21 Checkpoint Inhibitor Combinations Among Themselves and with Chemotherapy. J. Thorac. Oncol. 2019;14:S1166. doi: 10.1016/j.jtho.2019.09.119. [DOI] [Google Scholar]
- 62.Antonia S.J., López-Martin J.A., Bendell J., Ott P.A., Taylor M., Eder J.P., Jäger D., Pietanza M.C., Le D.T., de Braud F., et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (CheckMate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016;17:883–895. doi: 10.1016/S1470-2045(16)30098-5. [DOI] [PubMed] [Google Scholar]
- 63.Liu R.C., Consuegra G., Chou S., Fernandez Peñas P. Vitiligo-like depigmentation in oncology patients treated with immuno-therapies for nonmelanoma metastatic cancers. Clin. Exp. Dermatol. 2019;44:643–646. doi: 10.1111/ced.13867. [DOI] [PubMed] [Google Scholar]
- 64.Quaglino P., Marenco F., Osella-Abate S., Cappello N., Ortoncelli M., Salomone B., Fierro M.T., Savoia P., Bernengo M.G. Vitiligo is an independent favourable prognostic factor in stage III and IV metastatic melanoma patients: Results from a single-institution hospital-based observational cohort study. Ann. Oncol. 2009;21:409–414. doi: 10.1093/annonc/mdp325. [DOI] [PubMed] [Google Scholar]
- 65.Hua C., Boussemart L., Mateus C., Routier E., Boutros C., Cazenave H., Viollet R., Thomas M., Roy S., Benannoune N., et al. Association of Vitiligo with Tumor Response in Patients with Metastatic Melanoma Treated with Pembrolizumab. JAMA Dermatol. 2016;152:45–51. doi: 10.1001/jamadermatol.2015.2707. [DOI] [PubMed] [Google Scholar]
- 66.Postow M.A. Managing Immune Checkpoint-Blocking Antibody Side Effects. Am. Soc. Clin. Oncol. Educ. Book. 2015;35:76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 67.Wolner Z.J., Marghoob A.A., Pulitzer M.P., Postow M.A., Marchetti M.A. A case report of disappearing pigmented skin lesions as-sociated with pembrolizumab treatment for metastatic melanoma. Br. J. Dermatol. 2018;178:265–269. doi: 10.1111/bjd.15354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Manson G., Marabelle A., Houot R. Hair Repigmentation with Anti–PD-1 and Anti–PD-L1 Immunotherapy: A Novel Hypothesis. [(accessed on 9 March 2023)];JAMA Dermatol. 2018 154:113. doi: 10.1001/jamadermatol.2017.4421. Available online: https://pubmed.ncbi.nlm.nih.gov/29167871. [DOI] [PubMed] [Google Scholar]
- 69.Rivera N., Boada A., Bielsa M.I., Fernández-Figueras M.T., Carcereny E., Moran M.T., Ferrándiz C. Hair Repigmentation During Immuno-therapy Treatment with an Anti-Programmed Cell Death 1 and Anti-Programmed Cell Death Ligand 1 Agent for Lung Cancer. [(accessed on 9 March 2023)];JAMA Dermatol. 2017 153:1162–1165. doi: 10.1001/jamadermatol.2017.2106. Available online: https://pubmed.ncbi.nlm.nih.gov/28700789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Teulings H.-E., Limpens J., Jansen S.N., Zwinderman A.H., Reitsma J.B., Spuls P.I., Luiten R.M. Vitiligo-Like Depigmentation in Patients with Stage III-IV Melanoma Receiving Immunotherapy and Its Association with Survival: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2015;33:773–781. doi: 10.1200/JCO.2014.57.4756. [DOI] [PubMed] [Google Scholar]
- 71.Nakamura Y., Tanaka R., Asami Y., Teramoto Y., Imamura T., Sato S., Maruyama H., Fujisawa Y., Matsuya T., Fujimoto M., et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J. Dermatol. 2016;44:117–122. doi: 10.1111/1346-8138.13520. [DOI] [PubMed] [Google Scholar]
- 72.Freeman-Keller M., Kim Y., Cronin H., Richards A., Gibney G., Weber J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016;22:886–894. doi: 10.1158/1078-0432.CCR-15-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zarbo A., Belum V., Sibaud V., Oudard S., Postow M., Hsieh J., Motzer R., Busam K., Lacouture M. Immune-related alopecia (areata and universalis) in cancer patients receiving immune checkpoint inhibitors. Br. J. Dermatol. 2016;176:1649–1652. doi: 10.1111/bjd.15237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rittmeyer A., Barlesi F., Waterkamp D., Park K., Ciardiello F., von Pawel J., Gadgeel S.M., Hida T., Kowalski D.M., Dols M.C., et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): A phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. Erratum in Lancet 2017, 389, e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lacouture M., Sibaud V. Toxic Side Effects of Targeted Therapies and Immunotherapies Affecting the Skin, Oral Mucosa, Hair, and Nails. Am. J. Clin. Dermatol. 2018;19:31–39. doi: 10.1007/s40257-018-0384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dasanu C.A., Lippman S.M., Plaxe S.C. Persistently curly hair phenotype with the use of nivolumab for squamous cell lung cancer. [(accessed on 9 March 2023)];J. Oncol. Pharm. Pract. 2016 23:638–640. doi: 10.1177/1078155216674355. Available online: https://pubmed.ncbi.nlm.nih.gov/27824586. [DOI] [PubMed] [Google Scholar]
- 77.Jackson L.K., Johnson D.B., Sosman J.A., Murphy B.A., Epstein J.B. Oral health in oncology: Impact of immunotherapy. [(accessed on 9 March 2023)];Support. Care Cancer. 2014 23:1–3. doi: 10.1007/s00520-014-2434-6. Available online: https://pubmed.ncbi.nlm.nih.gov/25216852. [DOI] [PubMed] [Google Scholar]
- 78.Vigarios E., Epstein J.B., Sibaud V. Oral mucosal changes induced by anticancer targeted therapies and immune checkpoint in-hibitors. [(accessed on 9 March 2023)];Support Care Cancer. 2017 25:1713–1739. doi: 10.1007/s00520-017-3629-4. Available online: https://pubmed.ncbi.nlm.nih.gov/28224235. [DOI] [PubMed] [Google Scholar]
- 79.McDermott D.F., Sosman J.A., Sznol M., Massard C., Gordon M.S., Hamid O., Powderly J.D., Infante J.R., Fassò M., Wang Y.V., et al. Atezolizumab, an Anti–Programmed Death-Ligand 1 Antibody, in Metastatic Renal Cell Carcinoma: Long-Term Safety, Clinical Activity, and Immune Correlates from a Phase Ia Study. [(accessed on 9 March 2023)];J. Clin. Oncol. 2016 34:833–842. doi: 10.1200/JCO.2015.63.7421. Available online: https://pubmed.ncbi.nlm.nih.gov/26755520. [DOI] [PubMed] [Google Scholar]
- 80.Collins L.K., Chapman M.S., Carter J.B., Samie F.H. Cutaneous adverse effects of the immune checkpoint inhibitors. [(accessed on 9 March 2023)];Curr. Probl. Cancer. 2017 41:125–128. doi: 10.1016/j.currproblcancer.2016.12.001. Available online: https://pubmed.ncbi.nlm.nih.gov/28190531. [DOI] [PubMed] [Google Scholar]
- 81.Inno A., Metro G., Bironzo P., Grimaldi A.M., Grego E., DI Nunno V., Picasso V., Massari F., Gori S. Pathogenesis, Clinical Manifestations and Management of Immune Checkpoint Inhibitors Toxicity. [(accessed on 10 March 2023)];Tumori J. 2017 103:405–421. doi: 10.5301/tj.5000625. Available online: https://pubmed.ncbi.nlm.nih.gov/28497847. [DOI] [PubMed] [Google Scholar]
- 82.Topalian S.L., Sznol M., McDermott D.F., Kluger H.M., Carvajal R.D., Sharfman W.H., Brahmer J.R., Lawrence D.P., Atkins M.B., Powderly J.D., et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. [(accessed on 10 March 2023)];J. Clin. Oncol. 2014 32:1020–1030. doi: 10.1200/JCO.2013.53.0105. Available online: https://pubmed.ncbi.nlm.nih.gov/24590637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jour G., Glitza I.C., Ellis R.M., Torres-Cabala C.A., Tetzlaff M.T., Li J.Y., Nagarajan P., Huen A., Aung P.P., Ivan D., et al. Autoimmune dermatologic toxicities from immune checkpoint blockade with anti-PD-1 antibody therapy: A report on bullous skin eruptions. [(accessed on 10 March 2023)];J. Cutan. Pathol. 2016 43:688–696. doi: 10.1111/cup.12717. Available online: https://pubmed.ncbi.nlm.nih.gov/27086658. [DOI] [PubMed] [Google Scholar]
- 84.Voskens C.J., Goldinger S.M., Loquai C., Robert C., Kaehler K.C., Berking C., Bergmann T., Bockmeyer C.L., Eigentler T., Fluck M., et al. The Price of Tumor Control: An Analysis of Rare Side Effects of Anti-CTLA-4 Therapy in Metastatic Melanoma from the Ipilimumab Network. [(accessed on 10 March 2023)];PLoS ONE. 2013 8:e53745. doi: 10.1371/journal.pone.0053745. Available online: https://pubmed.ncbi.nlm.nih.gov/23341990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hwang S.J.E., Carlos G., Wakade D., Sharma R., Fernandez-Penas P. Ipilimumab-induced acute generalized exanthematous pus-tulosis in a patient with metastatic melanoma. [(accessed on 10 March 2023)];Melanoma Res. 2016 26:417–420. doi: 10.1097/CMR.0000000000000261. Available online: https://pubmed.ncbi.nlm.nih.gov/27031538. [DOI] [PubMed] [Google Scholar]
- 86.Saw S., Lee H.Y., Ng Q.S. Pembrolizumab-induced Stevens–Johnson syndrome in non-melanoma patients. [(accessed on 10 March 2023)];Eur. J. Cancer. 2017 81:237–239. doi: 10.1016/j.ejca.2017.03.026. Available online: https://pubmed.ncbi.nlm.nih.gov/28438440. [DOI] [PubMed] [Google Scholar]
- 87.Vivar K.L., Deschaine M., Messina J., Divine J.M., Rabionet A., Patel N., Harrington M.A., Seminario-Vidal L. Epidermal programmed cell death-ligand 1 expression in TEN associated with nivolumab therapy. [(accessed on 10 March 2023)];J. Cutan. Pathol. 2017 44:381–384. doi: 10.1111/cup.12876. Available online: https://pubmed.ncbi.nlm.nih.gov/28000240. [DOI] [PubMed] [Google Scholar]
- 88.Goldinger S.M., Stieger P., Meier B., Micaletto S., Contassot E., French L.E., Dummer R. Cytotoxic Cutaneous Adverse Drug Reactions during Anti-PD-1 Therapy. [(accessed on 10 March 2023)];Clin. Cancer Res. 2016 22:4023–4029. doi: 10.1158/1078-0432.CCR-15-2872. Available online: https://pubmed.ncbi.nlm.nih.gov/26957557. [DOI] [PubMed] [Google Scholar]
- 89.Lu J., Thuraisingam T., Chergui M., Nguyen K. Nivolumab-associated DRESS syndrome: A case report. [(accessed on 11 March 2023)];JAAD Case Rep. 2019 5:216–218. doi: 10.1016/j.jdcr.2018.11.017. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6374958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ai L., Gao J., Zhao S., Li Q., Cui Y.-H., Liu Q., Wu D., Wang Y., Jin X., Ji Y., et al. Nivolumab-associated DRESS in a genetic susceptible individual. [(accessed on 11 March 2023)];J. Immunother. Cancer. 2021 9:e002879. doi: 10.1136/jitc-2021-002879. Available online: https://pubmed.ncbi.nlm.nih.gov/34599025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen C.-B., Wu M.-Y., Ng C.Y., Lu C.-W., Wu J., Kao P.-H., Yang C.-K., Peng M.-T., Huang C.-Y., Chang W.-C., et al. Severe cutaneous adverse reactions induced by targeted anticancer therapies and immunotherapies. [(accessed on 10 March 2023)];Cancer Manag. Res. 2018 10:1259–1273. doi: 10.2147/CMAR.S163391. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5962313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Raschi E., Antonazzo I.C., La Placa M., Ardizzoni A., Poluzzi E., De Ponti F. Serious Cutaneous Toxicities with Immune Checkpoint Inhibitors in the U.S. Food and Drug Administration Adverse Event Reporting System. [(accessed on 10 March 2023)];Oncol. 2019 24:e1228–e1231. doi: 10.1634/theoncologist.2019-0250. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6853099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bs N.J.M., Ravi V., Cheng K., Bach D.Q., Worswick S. Stevens-Johnson syndrome and toxic epidermal necrolysis-like reactions to checkpoint inhibitors: A systematic review. [(accessed on 10 March 2023)];Int. J. Dermatol. 2020 59:e183–e188. doi: 10.1111/ijd.14811. Available online: https://pubmed.ncbi.nlm.nih.gov/32052409. [DOI] [PubMed] [Google Scholar]
- 94.Abdel-Wahab N., Shah M., Suarez-Almazor M.E. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. [(accessed on 11 March 2023)];PLoS ONE. 2016 11:e0160221. doi: 10.1371/journal.pone.0160221. Available online: https://pubmed.ncbi.nlm.nih.gov/27472273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Estenaga A., Rodriguez-Garijo N., Tomás-Velázquez A., Antoñanzas-Pérez J., Alvarez-Gigli M.L., García-Tobar L., Espaa-Alonso A., Salido-Vallejo R. Immuno-therapy-intensified paraneoplastic dermatomyositis. [(accessed on 11 March 2023)];Indian J. Dermatol. Venereol. Leprol. 2021 88:93–96. doi: 10.25259/IJDVL_1306_20. Available online: https://pubmed.ncbi.nlm.nih.gov/34491672. [DOI] [PubMed] [Google Scholar]
- 96.Thomas R., Patel H., Scott J. Dermatomyositis Flare with Immune Checkpoint Inhibitor Therapy for Melanoma. [(accessed on 11 March 2023)];Cureus. 2021 13:e14387. doi: 10.7759/cureus.14387. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8106943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Coustal C., Du Thanh A., Roubille F., Assenat E., Maria A.T. Rare cutaneous toxicity of immune checkpoint inhibitors: A case of durvalumab-induced dermatomyositis. [(accessed on 11 March 2023)];Eur. J. Cancer. 2021 155:25–27. doi: 10.1016/j.ejca.2021.06.031. Available online: https://pubmed.ncbi.nlm.nih.gov/34332401. [DOI] [PubMed] [Google Scholar]
- 98.Freites-Martinez A., Kwong B.Y., Rieger K.E., Coit D.G., Colevas A.D., Lacouture M.E. Eruptive Keratoacanthomas Associated with Pembrolizumab Therapy. [(accessed on 11 March 2023)];JAMA Dermatol. 2017 153:694–697. doi: 10.1001/jamadermatol.2017.0989. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5523926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seban R.-D., Vermersch C., Champion L., Bonsang B., Roger A., Ghidaglia J. Immune-Related Erythema Nodosum Mimicking in Transit Melanoma Metastasis on [18F]-FDG PET/CT. [(accessed on 11 March 2023)];Diagnostics. 2021 11:747. doi: 10.3390/diagnostics11050747. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8143543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pach J., Moody K., Ring N., Panse G., Zhang M., Deverapalli S., Leventhal J. Erythema nodosum-like panniculitis associated with immune checkpoint inhibitor therapy: Two cases reporting a rare cutaneous adverse event. [(accessed on 11 March 2023)];JAAD Case Rep. 2021 13:118–120. doi: 10.1016/j.jdcr.2021.05.002. Available online: https://pubmed.ncbi.nlm.nih.gov/34189226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tetzlaff M.T., Jazaeri A.A., Torres-Cabala C.A., Korivi B.R., Landon G.A., Nagarajan P., Choksi A., Chen L., Uemura M., Aung P.P., et al. Erythema nodosum-like panniculitis mimicking disease recurrence: A novel toxicity from immune checkpoint blockade therapy-Report of 2 patients. [(accessed on 11 March 2023)];J. Cutan. Pathol. 2017 44:1080–1086. doi: 10.1111/cup.13044. Available online: https://pubmed.ncbi.nlm.nih.gov/28901560. [DOI] [PubMed] [Google Scholar]
- 102.Munoz J., Guillot B., Girard C., Dereure O., Du-Thanh A. First report of ipilimumab-induced Grover disease. [(accessed on 11 March 2023)];Br. J. Dermatol. 2014 171:1236–1237. doi: 10.1111/bjd.13058. Available online: https://pubmed.ncbi.nlm.nih.gov/24749658. [DOI] [PubMed] [Google Scholar]
- 103.Uemura M., Faisal F., Haymaker C., McQuail N., Sirmans E., Hudgens C.W., Barbara L., Bernatchez C., Curry J.L., Hwu P., et al. A case report of Grover’s disease from immu-notherapy-a skin toxicity induced by inhibition of CTLA-4 but not PD-1. [(accessed on 11 March 2023)];J. Immunother. Cancer. 2016 4:55. doi: 10.1186/s40425-016-0157-6. Available online: https://pubmed.ncbi.nlm.nih.gov/27660709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Koelzer V.H., Buser T., Willi N., Rothschild S.I., Wicki A., Schiller P., Cathomas G., Zippelius A., Mertz K.D. Grover’s-like drug eruption in a patient with metastatic melanoma under ipilimumab therapy. [(accessed on 11 March 2023)];J. Immunother. Cancer. 2016 4:47. doi: 10.1186/s40425-016-0151-z. Available online: https://pubmed.ncbi.nlm.nih.gov/27532022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Welborn M.E., Kubicki S.L., Patel A.B. Pyoderma Gangrenosum Following Initiation of Immune Checkpoint Inhibitor Therapy. J. Immunother. Precis. Oncol. 2018;1:82–84. doi: 10.4103/JIPO.JIPO_11_18. [DOI] [Google Scholar]
- 106.Tsibris H., Lian C., Ho A. Pembrolizumab-associated pyoderma gangrenosum in a patient with metastatic squamous cell car-cinoma. Dermatol. Online J. 2021;27 doi: 10.5070/D3274053158. [DOI] [PubMed] [Google Scholar]
- 107.Lomax A.J., McGuire H.M., McNeil C., Choi C.J., Hersey P., Karikios D., Shannon K., van Hal S., Carr U., Crotty A., et al. Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: Case series and immunophenotypic analysis. [(accessed on 11 March 2023)];Int. J. Rheum. Dis. 2017 20:1277–1285. doi: 10.1111/1756-185X.13076. Available online: https://pubmed.ncbi.nlm.nih.gov/28480561. [DOI] [PubMed] [Google Scholar]
- 108.Suozzi K.C., Stahl M., Ko C.J., Chiang A., Gettinger S.N., Siegel M.D., Bunick C.G. Immune-related sarcoidosis observed in combination ipilimumab and nivolumab therapy. [(accessed on 11 March 2023)];JAAD Case Rep. 2016 2:264–268. doi: 10.1016/j.jdcr.2016.05.002. Available online: https://pubmed.ncbi.nlm.nih.gov/27486590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cotliar J., Raja N., Raz D., Boswell W.J., Chen R., Querfeld C. Pembrolizumab-associated sarcoidosis. [(accessed on 11 March 2023)];JAAD Case Rep. 2016 2:290–293. doi: 10.1016/j.jdcr.2016.06.004. Available online: https://pubmed.ncbi.nlm.nih.gov/27504482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tetzlaff M.T., Nelson K.C., Diab A., Staerkel G.A., Nagarajan P., Torres-Cabala C.A., Chasen B.A., Wargo J.A., Prieto V.G., Amaria R.N., et al. Granulomatous/sarcoid-like lesions asso-ciated with checkpoint inhibitors: A marker of therapy response in a subset of melanoma patients. [(accessed on 11 March 2023)];J. Immunother. Cancer. 2018 6:14. doi: 10.1186/s40425-018-0323-0. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5810034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gkiozos I., Kopitopoulou A., Kalkanis A., Vamvakaris I.N., Judson M.A., Syrigos K.N. Sarcoidosis-Like Reactions Induced by Checkpoint Inhibitors. [(accessed on 11 March 2023)];J. Thorac. Oncol. 2018 13:1076–1082. doi: 10.1016/j.jtho.2018.04.031. Available online: https://pubmed.ncbi.nlm.nih.gov/29763666. [DOI] [PubMed] [Google Scholar]
- 112.Cappelli L.C., Gutierrez A.K., Baer A.N., Albayda J., Manno R.L., Haque U., Lipson E.J., Bleich K.B., Shah A.A., Naidoo J., et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. [(accessed on 11 March 2023)];Ann. Rheum. Dis. 2016 76:43–50. doi: 10.1136/annrheumdis-2016-209595. Available online: https://pubmed.ncbi.nlm.nih.gov/27307501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yaşar H.A., Akkus E., Heper A.O., Akay B.N., Urun Y., Utkan G. Sweet’s syndrome under ipilimumab therapy and a brief comparison of the cases in literature. [(accessed on 11 March 2023)];J. Oncol. Pharm. Pract. 2020 26:1762–1764. doi: 10.1177/1078155220906885. Available online: https://pubmed.ncbi.nlm.nih.gov/32089071. [DOI] [PubMed] [Google Scholar]
- 114.Pintova S., Sidhu H., Friedlander P.A., Holcombe R.F. Sweet’s syndrome in a patient with metastatic melanoma after ipilimumab therapy. [(accessed on 11 March 2023)];Melanoma Res. 2013 23:498–501. doi: 10.1097/CMR.0000000000000017. Available online: https://pubmed.ncbi.nlm.nih.gov/24113862. [DOI] [PubMed] [Google Scholar]
- 115.Bousquet E., Zarbo A., Tournier E., Chevreau C., Mazieres J., Lacouture M., Sibaud V. Development of Papulopustular Rosacea during Nivolumab Therapy for Metastatic Cancer. [(accessed on 11 March 2023)];Acta Derm.-Venereol. 2017 97:539–540. doi: 10.2340/00015555-2566. Available online: https://pubmed.ncbi.nlm.nih.gov/27826614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gambichler T., Strutzmann S., Tannapfel A., Susok L. Paraneoplastic acral vascular syndrome in a patient with metastatic melanoma under immune checkpoint blockade. [(accessed on 11 March 2023)];BMC Cancer. 2017 17:327. doi: 10.1186/s12885-017-3313-6. Available online: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-017-3313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Le Burel S., Champiat S., Routier E., Aspeslagh S., Albiges L., Szwebel T.A., Michot J.-M., Chretien P., Mariette X., Voisin A.-L., et al. Onset of connective tissue disease following an-ti-PD1/PD-L1 cancer immunotherapy. [(accessed on 11 March 2023)];Ann. Rheum. Dis. 2018 77:468–470. doi: 10.1136/annrheumdis-2016-210820. Available online: https://pubmed.ncbi.nlm.nih.gov/28242618. [DOI] [PubMed] [Google Scholar]
- 118.Sanlorenzo M., Choudhry A., Vujic I., Posch C., Chong K., Johnston K., Meier M., Osella-Abate S., Quaglino P., Daud A., et al. Comparative profile of cutaneous adverse events: BRAF/MEK inhibitor combination therapy versus BRAF monotherapy in melanoma. [(accessed on 11 March 2023)];J. Am. Acad. Dermatol. 2014 71:1102–1109.e1. doi: 10.1016/j.jaad.2014.09.002. Available online: https://pubmed.ncbi.nlm.nih.gov/25440439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dummer R., Rinderknecht J., Goldinger S.M. Ultraviolet A and Photosensitivity during Vemurafenib Therapy. [(accessed on 11 March 2023)];N. Engl. J. Med. 2012 366:480–481. doi: 10.1056/NEJMc1113752. Available online: https://www.nejm.org/doi/full/10.1056/nejmc1113752. [DOI] [PubMed] [Google Scholar]
- 120.Boussemart L., Routier E., Mateus C., Opletalova K., Sebille G., Kamsu-Kom N., Thomas M., Vagner S., Favre M., Tomasic G., et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: A study of 42 patients. [(accessed on 11 March 2023)];Ann. Oncol. 2013 24:1691–1697. doi: 10.1093/annonc/mdt015. Available online: http://www.annalsofoncology.org/article/S0923753419373016/fulltext. [DOI] [PubMed] [Google Scholar]
- 121.Reyes-Habito C.M., Roh E.K. Cutaneous reactions to chemotherapeutic drugs and targeted therapy for cancer: Part II. Targeted therapy. [(accessed on 11 March 2023)];J. Am. Acad. Dermatol. 2014 71:217.e1–217.e11. doi: 10.1016/j.jaad.2014.04.013. Available online: https://pubmed.ncbi.nlm.nih.gov/25037801. [DOI] [PubMed] [Google Scholar]
- 122.Anforth R., Fernandez-Penas P. BRAF Inhibitor Induced Verrucal Keratosis. [(accessed on 11 March 2023)];Am. J. Dermatopathol. 2014 36:192. doi: 10.1097/DAD.0b013e3182858142. Available online: https://pubmed.ncbi.nlm.nih.gov/23435364. [DOI] [PubMed] [Google Scholar]
- 123.Anforth R., Carlos G., Clements A., Kefford R., Fernandez-Peñas P. Cutaneous adverse events in patients treated with BRAF inhibitor-based therapies for metastatic melanoma for longer than 52 weeks. [(accessed on 11 March 2023)];Br. J. Dermatol. 2014 172:239–243. doi: 10.1111/bjd.13200. Available online: https://pubmed.ncbi.nlm.nih.gov/25040674. [DOI] [PubMed] [Google Scholar]
- 124.Mattei P.L., Alora-Palli M.B., Kraft S., Lawrence D.P., Flaherty K.T., Kimball A.B. Cutaneous effects of BRAF inhibitor therapy: A case series. [(accessed on 11 March 2023)];Ann. Oncol. 2013 24:530–537. doi: 10.1093/annonc/mds292. Available online: https://pubmed.ncbi.nlm.nih.gov/23035153. [DOI] [PubMed] [Google Scholar]
- 125.Anforth R., Tembe V., Blumetti T., Fernandez-Peñas P. Mutational analysis of cutaneous squamous cell carcinomas and verrucal keratosis in patients taking BRAF inhibitors. [(accessed on 11 March 2023)];Pigment. Cell Melanoma Res. 2012 25:569–572. doi: 10.1111/j.1755-148X.2012.01031.x. Available online: https://pubmed.ncbi.nlm.nih.gov/22726224. [DOI] [PubMed] [Google Scholar]
- 126.Sosman J.A., Kim K.B., Schuchter L., Gonzalez R., Pavlick A.C., Weber J.S., McArthur G.A., Hutson T.E., Moschos S.J., Flaherty K.T., et al. Survival in BRAF V600–Mutant Advanced Melanoma Treated with Vemurafenib. [(accessed on 11 March 2023)];N. Engl. J. Med. 2012 366:707–714. doi: 10.1056/NEJMoa1112302. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa1112302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Anforth R., Blumetti T., Kefford R., Sharma R., Scolyer R., Kossard S., Long G., Fernandez-Peñas P. Cutaneous manifestations of dabrafenib (GSK2118436): A selective inhibitor of mutant BRAF in patients with metastatic melanoma. [(accessed on 11 March 2023)];Br. J. Dermatol. 2012 167:1153–1160. doi: 10.1111/j.1365-2133.2012.11155.x. Available online: https://pubmed.ncbi.nlm.nih.gov/22804352. [DOI] [PubMed] [Google Scholar]
- 128.Cohen P.R., Bedikian A.Y., Kim K.B. Appearance of New Vemurafenib-associated Melanocytic Nevi on Normal-appearing Skin: Case Series and a Review of Changing or New Pigmented Lesions in Patients with Metastatic Malignant Melanoma After Ini-tiating Treatment with Vemurafenib. [(accessed on 12 March 2023)];J. Clin. Aesthet. Dermatol. 2013 6:27. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3662681. [PMC free article] [PubMed] [Google Scholar]
- 129.Haenssle H.A., Kraus S.L., Brehmer F., Kretschmer L., Völker B., Asper H., Kapp A., Gutzmer R. Dynamic changes in nevi of a patient with melanoma treated with vemurafenib: Importance of sequential dermoscopy. [(accessed on 12 March 2023)];Arch. Dermatol. 2012 148:1183–1185. doi: 10.1001/archdermatol.2012.2649. Available online: https://pubmed.ncbi.nlm.nih.gov/22911096. [DOI] [PubMed] [Google Scholar]
- 130.Meneguzzo A., Lazzarotto A., Alaibac M. Eruptive Melanocytic Nevi Secondary to Encorafenib for BRAF Mutant Metastatic Colorectal Cancer. [(accessed on 12 March 2023)];Vivo. 2019 34:441–445. doi: 10.21873/invivo.11793. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6984112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anforth R.M., Carlos G.R., Scolyer R.A., Chou S., Fernandez-Peñas P. Eruptive naevi in a patient treated with LGX818 for BRAF mutant metastatic melanoma. [(accessed on 12 March 2023)];Melanoma Res. 2015 25:91–94. doi: 10.1097/CMR.0000000000000127. Available online: https://pubmed.ncbi.nlm.nih.gov/25380183. [DOI] [PubMed] [Google Scholar]
- 132.Zimmer L., Hillen U., Livingstone E., Lacouture M.E., Busam K., Carvajal R.D., Egberts F., Hauschild A., Kashani-Sabet M., Goldinger S.M., et al. Atypical Melanocytic Proliferations and New Primary Melanomas in Patients with Advanced Melanoma Undergoing Selective BRAF Inhibition. [(accessed on 12 March 2023)];J. Clin. Oncol. 2012 30:2375–2383. doi: 10.1200/JCO.2011.41.1660. Available online: https://pubmed.ncbi.nlm.nih.gov/22614973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Morita H., Nagai R. Vemurafenib in melanoma with BRAF V600E mutation. [(accessed on 12 March 2023)];N. Engl. J. Med. 2011 365:1448. doi: 10.1056/NEJMc1108651. author reply 1450. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21995398. [DOI] [PubMed] [Google Scholar]
- 134.Robert C., Karaszewska B., Schachter J., Rutkowski P., Mackiewicz A., Stroiakovski D., Lichinitser M., Dummer R., Grange F., Mortier L., et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. [(accessed on 12 March 2023)];N. Engl. J. Med. 2015 372:30–39. doi: 10.1056/NEJMoa1412690. Available online: https://pubmed.ncbi.nlm.nih.gov/25399551. [DOI] [PubMed] [Google Scholar]
- 135.Dummer R., Ascierto P.A., Gogas H.J., Arance A., Mandala M., Liszkay G., Garbe C., Schadendorf D., Krajsova I., Gutzmer R., et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. [(accessed on 12 March 2023)];Lancet Oncol. 2018 19:1315–1327. doi: 10.1016/S1470-2045(18)30497-2. Available online: https://pubmed.ncbi.nlm.nih.gov/30219628. [DOI] [PubMed] [Google Scholar]
- 136.Piraccini B.M., Patrizi A., Fanti P.A., Starace M., Bruni F., Melotti B., Misciali C., Dika E. RASopathic alopecia: Hair changes associated with vemu-rafenib therapy. [(accessed on 12 March 2023)];J. Am. Acad. Dermatol. 2015 72:738–741. doi: 10.1016/j.jaad.2015.01.011. Available online: http://www.jaad.org/article/S019096221500050X/fulltext. [DOI] [PubMed] [Google Scholar]
- 137.Cavalieri S., Di Guardo L., Cossa M., Cimminiello C., Del Vecchio M. Unusual Skin Carcinomas Induced by BRAF Inhibitor for Metastatic Melanoma: A Case Report. [(accessed on 12 March 2023)];J. Clin. Diagn. Res. 2017 11:XD06–XD08. doi: 10.7860/JCDR/2017/26881.10200. Available online: https://pubmed.ncbi.nlm.nih.gov/28893027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hui Ong E.L., Sinha R., Jmor S., Fearfield L. BRAF Inhibitor-Associated Granulomatous Dermatitis: A Report of 3 Cases. Am. J. Dermatopathol. 2019;41:214–217. doi: 10.1097/DAD.0000000000001276. [DOI] [PubMed] [Google Scholar]
- 139.Garrido M.C., Gutierrez C., Riveiro-Falkenbach E., Ortiz P., Rodriguez-Peralto J.L. BRAF Inhibitor-Induced Antitumoral Granu-lomatous Dermatitis Eruption in Advanced Melanoma. [(accessed on 13 March 2023)];Am. J. Dermatopathol. 2015 37:795–798. doi: 10.1097/DAD.0000000000000281. Available online: https://pubmed.ncbi.nlm.nih.gov/26381028. [DOI] [PubMed] [Google Scholar]
- 140.Park J.J., Hawryluk E.B., Tahan S.R., Flaherty K., Kim C.C. Cutaneous granulomatous eruption and successful response to potent topical steroids in patients undergoing targeted BRAF inhibitor treatment for metastatic melanoma. [(accessed on 13 March 2023)];JAMA Dermatol. 2014 150:307–311. doi: 10.1001/jamadermatol.2013.7919. Available online: https://pubmed.ncbi.nlm.nih.gov/24352115. [DOI] [PubMed] [Google Scholar]
- 141.Chu E.Y., Wanat K.A., Miller C.J., Amaravadi R.K., Fecher L.A., Brose M.S., McGettigan S., Giles L.R., Schuchter L.M., Seykora J.T., et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: A clinicopathologic study. [(accessed on 13 March 2023)];J. Am. Acad. Dermatol. 2012 67:1265–1272. doi: 10.1016/j.jaad.2012.04.008. Available online: https://pubmed.ncbi.nlm.nih.gov/22609219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lilly E., Burke M., Kluger H., Choi J. Pregabalin for the Treatment of Painful Hand-Foot Skin Reaction Associated with Dabrafenib. [(accessed on 12 March 2023)];JAMA Dermatol. 2015 151:102. doi: 10.1001/jamadermatol.2014.2455. Available online: https://jamanetwork.com/journals/jamadermatology/fullarticle/1910311. [DOI] [PubMed] [Google Scholar]
- 143.Vázquez-Osorio I., Sánchez-Aguilar M.D., García-Rodiño S., Suárez-Peñaranda J.M., Aliste C., Vázquez-Veiga H. Vemuraf-enib-induced neutrophilic panniculitis: A new case and review of the literature. Am. J. Dermatopathol. 2016;38:e93–e96. doi: 10.1097/DAD.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 144.Monfort J.-B., Pagès C., Schneider P., Neyns B., Comte C., Bagot M., Vignon-Pennamen M.-D., Viguier M., Lebbé C. Vemurafenib-induced neutrophilic panniculitis. [(accessed on 13 March 2023)];Melanoma Res. 2012 22:399–401. doi: 10.1097/CMR.0b013e3283570792. Available online: https://pubmed.ncbi.nlm.nih.gov/22828248. [DOI] [PubMed] [Google Scholar]
- 145.Alonso-Castro L., Ríos-Buceta L., Vano-Galvan S., Moreno C., Soria-Rivas A., Jaén P. Vitiligo in 2 patients receiving vemurafenib for metastatic melanoma. [(accessed on 13 March 2023)];J. Am. Acad. Dermatol. 2013 69:e28–e29. doi: 10.1016/j.jaad.2013.01.012. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23768302. [DOI] [PubMed] [Google Scholar]
- 146.Nasca M.R., Lacarrubba F., Ferraù F., Micali G. Vitiligo of the Face in a Patient Treated with Vemurafenib for Metastatic Melanoma. [(accessed on 13 March 2023)];J. Drugs Dermatol. 2016 15:766–768. Available online: https://pubmed.ncbi.nlm.nih.gov/27272087. [PubMed] [Google Scholar]
- 147.Jänne P.A., Shaw A.T., Pereira J.R., Jeannin G., Vansteenkiste J., Barrios C., Franke F.A., Grinsted L., Zazulina V., Smith P., et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: A randomised, multicentre, placebo-controlled, phase 2 study. [(accessed on 13 March 2023)];Lancet Oncol. 2013 14:38–47. doi: 10.1016/S1470-2045(12)70489-8. Available online: https://pubmed.ncbi.nlm.nih.gov/23200175. [DOI] [PubMed] [Google Scholar]
- 148.Balagula Y., Huston K.B., Busam K.J., Lacouture M.E., Chapman P.B., Myskowski P.L. Dermatologic side effects associated with the MEK 1/2 inhibitor selumetinib (AZD6244, ARRY-142886) [(accessed on 13 March 2023)];Investig. New Drugs. 2011 29:1114. doi: 10.1007/s10637-010-9567-3. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5691597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Volontè M., Isoletta E., Gordon S., Foiadelli T., Bassanese F., Rossi A., Marseglia G.L., Savasta S., Brazzelli V. Acneiform rash as a side effect of selumetinib in a child with neurofibromatosis type 1 treated for inoperable plexiform neurofibromas: Good results with doxycycline. [(accessed on 13 March 2023)];Dermatol. Ther. 2022 35:e15607. doi: 10.1111/dth.15607. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9541585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smith T.M., Gilliland K., Clawson G.A., Thiboutot D. IGF-1 Induces SREBP-1 Expression and Lipogenesis in SEB-1 Sebocytes via Activation of the Phosphoinositide 3-Kinase/Akt Pathway. J. Investig. Dermatol. 2008;128:1286–1293. doi: 10.1038/sj.jid.5701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Patel U., Cornelius L., Anadkat M.J. MEK inhibitor-induced dusky erythema: Characteristic drug hypersensitivity manifestation in 3 patients. JAMA Dermatol. 2015;151:78–81. doi: 10.1001/jamadermatol.2014.3207. [DOI] [PubMed] [Google Scholar]
- 152.Carlberg V.M., Davies O.M.T., Brandling-Bennett H.A., Leary S.E.S., Huang J.T., Coughlin C.C., Gupta D. Cutaneous reactions to pediatric cancer treatment part II: Targeted therapy. [(accessed on 13 March 2023)];Pediatr. Dermatol. 2020 38:18–30. doi: 10.1111/pde.14495. Available online: https://pubmed.ncbi.nlm.nih.gov/33378085. [DOI] [PubMed] [Google Scholar]
- 153.Hwang S.J.E., Anforth R., Carlos G., Fernandez-Peñas P. Cutaneous Adverse Events of New Anti-melanoma Therapies: Classification and Management. [(accessed on 14 March 2023)];Actas Dermosifiliogr. 2017 108:6–16. doi: 10.1016/j.ad.2016.05.019. Available online: https://pubmed.ncbi.nlm.nih.gov/27642030. [DOI] [PubMed] [Google Scholar]
- 154.Flaherty K.T., Robert C., Hersey P., Nathan P., Garbe C., Milhem M., Demidov L.V., Hassel J.C., Rutkowski P., Mohr P., et al. Improved Survival with MEK Inhibition in BRAF-Mutated Melanoma. [(accessed on 14 March 2023)];N. Engl. J. Med. 2012 367:107–114. doi: 10.1056/NEJMoa1203421. Available online: https://pubmed.ncbi.nlm.nih.gov/22663011. [DOI] [PubMed] [Google Scholar]
- 155.Dombi E., Baldwin A., Marcus L.J., Fisher M.J., Weiss B., Kim A., Whitcomb P., Martin S., Aschbacher-Smith L.E., Rizvi T.A., et al. Activity of Selumetinib in Neurofibromatosis Type 1–Related Plexiform Neurofibromas. [(accessed on 14 March 2023)];N. Engl. J. Med. 2016 375:2550–2560. doi: 10.1056/NEJMoa1605943. Available online: https://pubmed.ncbi.nlm.nih.gov/28029918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Song H., Zhong C.S., Kieran M.W., Chi S.N., Wright K.D., Huang J.T. Cutaneous reactions to targeted therapies in children with CNS tumors: A cross-sectional study. [(accessed on 14 March 2023)];Pediatr. Blood Cancer. 2019 66:e27682. doi: 10.1002/pbc.27682. Available online: https://pubmed.ncbi.nlm.nih.gov/30821092. [DOI] [PubMed] [Google Scholar]
- 157.Alkeraye S., Maire C., Desmedt E., Templier C., Mortier L. Persistent alopecia induced by vismodegib. Br. J. Dermatol. 2015;172:1671–1672. doi: 10.1111/bjd.13630. [DOI] [PubMed] [Google Scholar]
- 158.Puig S., Serra-Guillén C., Pérez-Pastor G., Martínez-Domenech Á., Cabrera R.F.-D. Experience with sonidegib in patients with advanced basal cell carcinoma: Case reports. [(accessed on 17 May 2023)];Drugs Context. 2022 11:1–8. doi: 10.7573/dic.2022-3-8. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9132536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fife K., Herd R., Lalondrelle S., Plummer R., Strong A., Jones S., Lear J.T. Managing adverse events associated with vismodegib in the treatment of basal cell carcinoma. Futur. Oncol. 2017;13:175–184. doi: 10.2217/fon-2016-0296. [DOI] [PubMed] [Google Scholar]
- 160.Reversible Cutaneous Side Effects of Vismodegib Treatment|MDedge Dermatology. [(accessed on 15 March 2023)]. Available online: https://www.mdedge.com/dermatology/article/133890/hair-nails/reversible-cutaneous-side-effects-vismodegib-treatment.
- 161.Le Moigne M., Saint-Jean M., Jirka A., Quéreux G., Peuvrel L., Brocard A., Gaultier A., Khammari A., Darmaun M., Dréno B. Dysgeusia and weight loss under treatment with vismodegib: Benefit of nutritional management. Support. Care Cancer. 2015;24:1689–1695. doi: 10.1007/s00520-015-2932-1. [DOI] [PubMed] [Google Scholar]
- 162.Yang H., Cong W.-N., Yoon J.S., Egan J.M. Vismodegib, an antagonist of hedgehog signaling, directly alters taste molecular signaling in taste buds. [(accessed on 18 March 2023)];Cancer Med. 2015 4:245–252. doi: 10.1002/cam4.350. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4329008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moreno-Arrones O.M., Carrillo-Gijon R., Sendagorta E., Rios-Buceta L. Acute generalized exanthematous pustulosis simulating Stevens-Johnson syndrome/toxic epidermal necrolysis associated with the use of vismodegib. JAAD Case Rep. 2018;4:123–125. doi: 10.1016/j.jdcr.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Caplash G., Curragh D.S., Halliday L., Huilgol S.C., Selva D. Report of cutaneous side effects of vismodegib treatment. [(accessed on 18 March 2023)];Clin. Exp. Ophthalmol. 2019 48:123–125. doi: 10.1111/ceo.13651. Available online: https://pubmed.ncbi.nlm.nih.gov/31569297. [DOI] [PubMed] [Google Scholar]
- 165.Aasi S., Silkiss R., Tang J.Y., Wysong A., Liu A., Epstein E., Oro A.E., Chang A.L.S. New Onset of Keratoacanthomas After Vismodegib Treatment for Locally Advanced Basal Cell Carcinomas: A Report of 2 Cases. [(accessed on 18 March 2023)];JAMA Dermatol. 2013 149:242–243. doi: 10.1001/jamadermatol.2013.1798. Available online: https://pubmed.ncbi.nlm.nih.gov/23426496. [DOI] [PMC free article] [PubMed] [Google Scholar]