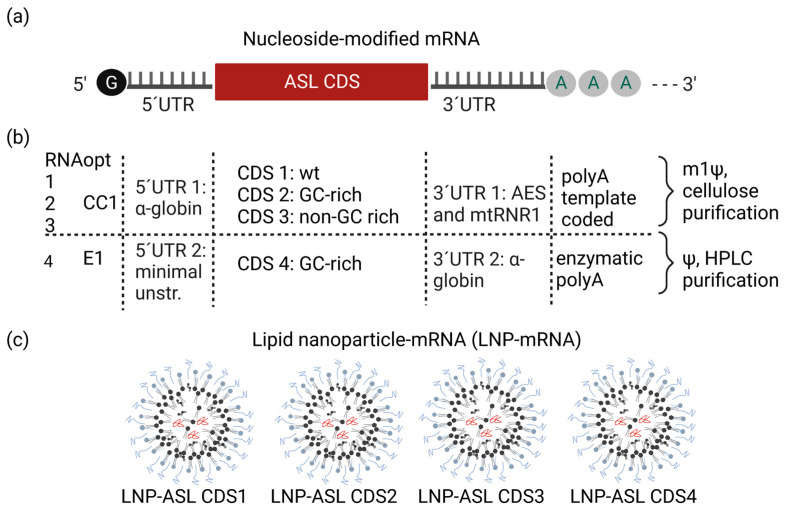
Figure 1.
Development of mRNA-LNP therapeutic for treatment of ASA. (a) Schematic shows optimized nucleoside-modified mRNA composition: a cap structure, 5′ and 3′ untranslated regions, ASL coding sequence (CDS) as well as poly(A); (b) table compares the differences of mRNA constructs used for the experiments. CleanCap1 (CC1) was used for CDS 1, 2, and 3 or enzymatic cap1 (E1) for CDS4, 5′UTR 1/3′UTR 1 was used in CDS 1-3, 5′ UTR 2 (minimal unstructured UTR)/3′UTR 2 in CDS 4. CDS 1–3 include an encoded polyA tail and incorporated N1-methylpseudouridine (m1Ψ) instead of uridine (U), whereas CDS4 was enzymatically polyA tailed and includes incorporated pseudouridine (Ψ) instead of uridine (U). CDS1 represents the wild-type coding sequence (wt.) while CDS2-CDS4 are GC-rich or non-GC-rich optimized coding sequences; (c) each of the four modified mRNAs was formulated individually in the same type of lipid nanoparticle (LNP), forming an mRNA therapeutic for the treatment of ASA.