Table 1.
Yields and enantioselectivities for bromoetherification of 1 using representative FDHsa
| ||||
|---|---|---|---|---|
| Entry | FDH | Time (h) | Yield (%)a | e.r.b |
| 1 | 4V+S | 18 | 66 | 79:21 |
| 2 | 6TL T52V | 18 | 48 | 36:64 |
| 3 | 4PL | 18 | 68 | 91:9 |
| 4 | 4PL | 2 | 88 | 91:9 |
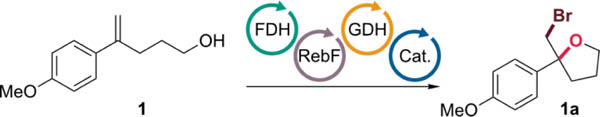
Reactions conducted using 1 mM 1, 5 mM NaBr, 5 mol% FDH, 100 μM NAD and FAD, 20 mM glucose, 1 mM glutathione, 2.5 μM flavin reductase (RebF),31 9 U/mL glucose dehydrogenase (GDH), and 35 U/mL catalase (Cat.) in 200 mM tricine, pH 7.5.
Yields determined by LC/MS relative to internal standard; selectivities determined by chiral HPLC.
