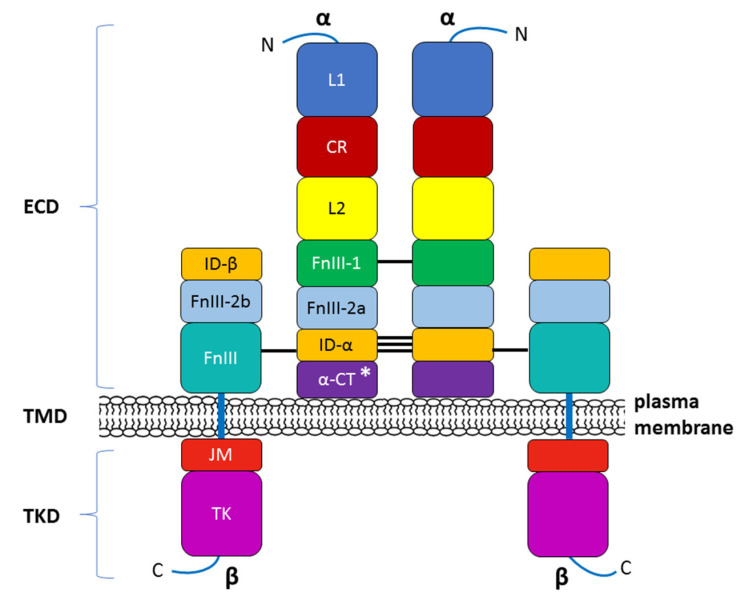
Figure 3.
Modular structure of the insulin receptor (INSR). The INSR is a preformed, covalently linked tetramer with two extracellular α subunits and two cell membrane-penetrating, tyrosine kinase-containing β subunits. The transmembrane localization of the receptor corresponds to the presence of the ectodomain (ECD) and the transmembrane (TMD) and tyrosine kinase (TKD) domains. The binding of insulin or IGF-1 to the dimeric ECD triggers auto-phosphorylation of the TKD and subsequent activation of downstream signaling molecules. On the left half of the INSR, spans of recognized protein modules whose boundaries typically correspond to the boundaries of the 22 exon-encoded sequences detected within the α and β subunits (not shown in this figure). N—NH2-terminus of the polypeptide chain; L1, L2—two large, leucine-rich repeat domains; CR—cysteine-rich region (domain); FnIII-1, FnIII-2a, FnIII-2b, FnIII-3—fibronectin type III domains; ID-α, ID-β—α or β subunit insert domain in FnIII-2 (the splitting into FnIII2a and FnIII2b), respectively; α-CT—C-terminus of the α subunit; JM—juxtamembrane domain; TK—tyrosine kinase domain; C—C-terminal tail. The two α subunits are linked by a disulfide bond between the two Cys 524 in FnIII-1. One to three of the triplet Cys at 682, 683, and 685 in the ID-α within FnIII-2 are also involved in α–α disulfide bridges. There is a single disulfide bridge between the α and β subunits between Cys 647 in the ID-α and Cys 872 in FnIII-3 (nomenclature of the B isoform) [64]. * The alternative splicing of exon 11 encodes a 12-amino acid sequence at the C-terminus of the α subunit in the INSR gene during transcription, resulting in the formation of the isoforms INSR-A and INSR-B.