Abstract
Background and aim. The nature of the association between obesity and poor prognosis of COVID-19 without the evaluation of other co-pathologies associated has not yet been clearly evaluated. The aim of the present pair-matched case–control study was to investigate the outcome of patients with SARS-CoV-2 infection in obese and non-obese patients matched considering gender, age, number of comorbidities, and Charlson Comorbidity Index. Methods. All the adults hospitalized for SARS-CoV-2 infection and with BMI ≥ 30 kg/m2 were included (Cases). For each Case, two patients with BMI < 30 kg/m2 pair matched for gender, age (±5 years), number of comorbidities (excluding obesity), and Charlson Comorbidity Index (±1) were enrolled (Controls). Results. Of the 1282 patients with SARS-CoV-2 infection followed during the study period, 141 patients with obesity and 282 patients without were enrolled in the case and control groups, respectively. Considering matching variables, there was no statistical difference between the two groups. Patients in the Control group developed more frequently a mild–moderate disease (67% vs. 46.1%, respectively), whereas obese patients were more prone to need intensive care treatment (41.8% vs. 26.6%, respectively; p = 0.001). Moreover, the prevalence of death during hospitalization was higher in the Case group than in the Control group (12.1% vs. 6.4%, p = 0.046). Discussion. We confirmed an association between obesity and severe outcome of patients with COVID-19, also considering other factors associated with a severe outcome of COVID-19. Thus, in the case of SARS-CoV-2 infection, the subjects with BMI ≥ 30 kg/m2 should be evaluated for early antiviral treatment to avoid the development of a severe course.
Keywords: obesity, COVID-19, SARS-CoV-2 infection, severity of disease, mortality
1. Introduction
In 2019, an emerging viral infection caused by a novel coronavirus named SARS-CoV-2 was reported in Wuhan (China). It rapidly spread worldwide, and on 12 March 2020, the World Health Organization (WHO) declared it a pandemic. Italy was the first European country hit by the infection, with the first autochthonous cases registered in Lombardy, a region in the north of the country. By the beginning of March 2020, there were more than 609,247,113 cases and over 6,503,894 deaths worldwide, with these numbers continuing to increase [1].
Coronavirus Infectious Disease-19 (COVID-19) is a multisystemic disorder characterized by a variety of symptoms ranging from non-severe to severe presentation [2]. Both clinical and laboratory parameters have been associated with a non-favorable outcome, such as older age, male sex, and several chronic diseases, including diabetes, hypertension, cardiovascular and renal disease, oncohematological disorders, and dementia [3,4,5,6]. Obesity is a chronic disease characterized by abnormal or excessive fat accumulation; this condition, defined as a body mass index (BMI) > 30 kg/m2 according to the WHO, leads to an increased risk of developing several chronic diseases, such as diabetes; cardiovascular diseases (CVDs); depression; non-alcoholic fatty liver disease; several infections, both viral and bacterial; colon diverticulosis; dysbiosis [7]; cancer; and it has also been associated with a significant reduction in life expectancy. Moreover, some studies showed that the risk of cardiovascular disease increased with the increase in BMI class (Class I, BMI 30–34.9 kg/m2; Class II, 35–39.9 kg/m2; Class III, >40 kg/m2 and waist circumference) [8,9,10]. During the past 50 years, obesity has become an increasing condition all over the world. The Global Burden of Disease (GDB) reported that one-third of the world population is currently classified as overweight or obese [11], and according to the State of Food Security and Nutrition in the World, obesity reached a global adult obesity rate of 13.2% in 2019 [12]. The Italian scenario is very similar to the rest of the world; in 2021, 46.2% of the Italian population was characterized by higher body weight, with a prevalence of overweight of 34.2% and obesity of 12% [13].
A condition of increased adipose tissue, especially visceral fat, predisposes to increased morbidity and mortality in patients with SARS-CoV-2 infection, as was also reported for influenza A [14,15]. However, obese subjects more frequently have other chronic conditions, for example, cardiovascular (CVD), liver or kidney disease, and arterial hypertension, which are all associated with a more severe clinical presentation of COVID-19. However, the nature of the association between obesity and poor prognosis of COVID-19 without the evaluation of other associated co-pathologies has not yet been clearly evaluated.
The aim of the present pair-matched case–control study was to investigate the outcome of patients with SARS-CoV-2 infection and obesity, compared with patients with a body mass index < 30 kg/m2, but with the same sex, similar age, presence of comorbidities, and Charlson Comorbidity Index, to assess the impact on COVID-19 severity and mortality.
2. Materials and Methods
2.1. Study Design and Setting
We performed a multicenter, observational, 1:2 matched case–control study involving nine COVID-19 units in eight cities in the Campania region in southern Italy: Naples, Caserta, Salerno, Benevento, Avellino, Pozzuoli, Eboli, and Vallo della Lucania. The patients were adults (≥18 years), hospitalized with a diagnosis of SARS-CoV-2 infection confirmed by a positive reverse transcriptase–polymerase chain reaction (RT-PCR) on a naso-oropharyngeal swab. The study period was from 28 February 2020 to 31 May 2021. The Campania COVID-19 cohort was an observational retrospective, prospective cohort in which all the infectious diseases units and structures that have faced the COVID-19 pandemic in Campania, a region in Southern Italy, have participated and participate. All patients admitted to one of the identified hospital units and confirmed positive to a RT-PCR for SARS-CoV-2 on a naso-oropharyngeal swab were eligible to be enrolled. Inclusion criteria were adults (>18 years old); accept and sign informed consent. Exclusion criteria were patients less than 18 years old; refused the informed consent. All patients were followed up since discharge for hospital or death during hospitalization. The database created for the CoviCamp cohort included all demographic, clinical, and laboratory data relevant for the aim. No study protocol or guidelines regarding the criteria of hospitalization were shared among the centers involved in the study, and the patients were hospitalized following the decision of physicians of each center. The details of the Campania COVID-19 cohort (CoViCamp cohort) were reported in previous studies [4,5,16,17,18].
For the present study, all patients with BMI ≥ 30 kg/m2 in the CoViCamp cohort were enrolled as Cases (Case group). For each Case, 2 patients with BMI < 30 kg/m2, pair matched for gender, age (±5 years), number of comorbidities (excluding obesity), and Charlson Comorbidity Index (CCI, ± 1), were chosen from the CoviCamp cohort (Control group). Exclusion criteria included minority age, and lack of clinical data and/or informed consent.
The study was approved by the Ethics Committee of the University of Campania L. Vanvitelli, Naples (No. 10877/2020). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study. This study was reported following the STROBE recommendations for an observational study (Supplementary Table S1).
2.2. Data Collection
All demographic, clinical, and laboratory details of both Cases and Controls were collected in a database. From this database, we extrapolated the data. The variable included in this study were number of patients with obesity (423 valid values), gender (423 valid values), number of patients with arterial hypertension (423 valid values), age (423 valid values), days from admission to discharge (422 valid values), number of comorbidity for each patients (423 valid values), Charlson Comorbidity Index (CCI) (417 valid values), number of patients with cardiovascular disease (423 valid values), number of patients with Chronic Obstructive Pulmonary Disease (COPD) (423 valid values), number of patients with chronic kidney disease (CKD) (423 valid values), number of patients with active cancer (423 valid values), number of patients with hepatopathy (421 valid values), number of patients with diabetes (423 valid values), number of patients with dementia (421 valid values), number of patients with fever (420 valid values), number of patients with dyspnea (419 valid values), number of patients with asthenia (418 valid values), number of patients with ageusia/dysgeusia (418 valid values), number of patients with anosmia/hyposmia (418 valid values), number of patients with diarrhea (418 valid values), number of patients with skin lesion (413 valid values), patients dead during hospitalization (423 valid values), patients with severe outcome or dead during hospitalization (423 valid values), white blood cell count at admission (WBC) (405 valid value), International Normalized ratio (INR) value at admission (376 valid value), lactate dehydrogenase (LDH) value at admission (377 valid value), creatinine value at admission (393 valid values), creatine phosphokinase (CPK) value at admission (274 valid values) aspartate aminotransferase (AST) value at admission (396 valid values), alanine aminotransferase (ALT) value at admission (383 valid values), total bilirubin value at admission (370 valid values), and PaO2/FiO2 (P/F) value at admission (378 valid values).
2.3. Definitions
The microbiological diagnosis of SARS-CoV-2 infection was defined as a positive RT-PCR test on a naso-oropharyngeal swab. Viral RNA was extracted from the naso-oropharyngeal swab with QIAamp Viral RNA Kits (Qiagen GmbH, Hilden, Germany); the detection of SARS-CoV-2 was performed by RT-PCR test using Bosphore® Novel Coronavirus (Anatolia Diagnostics and Biotechnology Products Inc., İstanbul, Turkey) Detection Kit V3, by primers designed on three viral regions: E, ORF1ab, and N regions. Variables included in this study and relative numbers are reported in the Supplementary Data. Patients who had BMI ≥ 30 kg/m2 were defined as obese in our database, and patients with BMI < 30 kg/m2 were defined as non-obese in our database.
Patients were followed until SARS-CoV-2-RNA negativity at naso-oropharyngeal swab or discharged from hospital or death. We defined patients with a mild, moderate, or severe outcome according to the clinical presentation of COVID-19. Precisely, patients with a mild outcome did not need oxygen (O2) therapy and/or had a MEWS score below 3 points. Patients with a moderate outcome required non-invasive O2 therapy and/or had a MEWS score equal to or above 3 points (≥3). Lastly, patients with a severe outcome needed management in an intensive care unit (ICU) and/or mechanical ventilation (invasive or not invasive); in this definition, we also included patients who died during hospitalization.
2.4. Statistical Analysis
For the descriptive analysis, categorical variables were presented as absolute numbers and their relative frequencies. Continuous variables were summarized as mean and standard deviation or as median and first and third interquartile value (Q1–Q3). We performed a comparison of patients with BMI ≥ 30 kg/m2 and with BMI < 30 kg/m2 using the Pearson chi-square or Fisher’s exact test for categorical variables and Student’s t- or Mann–Whitney tests for continuous variables. Student’s t-test was used for parametric variables and the Mann–Whitney test for non-parametric variables. To evaluate the distribution of continuous variables, we used the Shapiro–Wilk test. To estimate the survival function, we used log-rank and the Kaplan–Meier curve for a visual representation. A value of p < 0.05 was considered statistically significant. Analyses were performed using STATA [19].
3. Results
During the study period, 2054 patients with SARS-CoV-2 infection were enrolled in the CoViCamp cohort. For 1282 patients, the status of obesity was reported, and they were enrolled in the present study. No difference in the demographic, clinical, or biochemical data was observed between the 282 patients for whom the datum of obesity was available and for the 772 without the obesity data. Only 18 of 1282 (1.4%) patients had been vaccinated against SARS-CoV-2 with a full schedule (two doses).
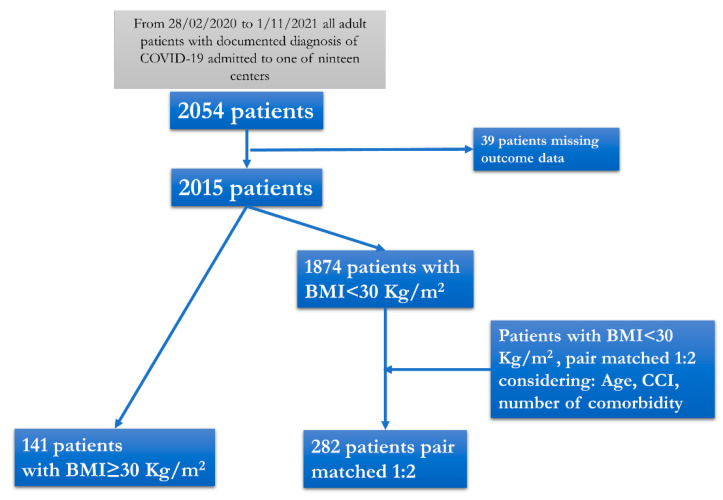
Of the 1282 patients enrolled, 141 (11%) presented at admission with BMI ≥ 30 kg/m2 and were included in the Case group, while 282 patients, pair matched for sex, age, CCI, and number of co-pathologies, but with BMI < 30 kg/m2, were included in the Control group (Figure 1).
Figure 1.
Flow chart of the patients included in the study.
Table 1 shows the demographic and clinical characteristics of the patients in both the Case and Control groups. Most patients were males (67.4%) in both the Case and Control groups, with a mean age of 58 (Q1–Q3 48–69) (Table 1). Obese patients more frequently showed arterial hypertension compared to the Control group (61% vs. 49.6%, p = 0.027) (Table 1); instead, no difference between the Cases and Controls was observed in the prevalence of other chronic diseases, such as CVDs, diabetes, chronic liver, and kidney disease (Table 1).
Table 1.
Demographic and clinical parameters in patients in the Case and Control groups.
| Case Group | Control Group | p-Value | |
|---|---|---|---|
| No. of patients | 141 | 282 | |
| Males, No. (%) | 95 (67.4) | 190(67.4) | 1 a |
| Age, years, median (Q1–Q3) | 58 (48–69) | 58 (48–69) | 0.925 b |
| Charlson comorbidity index, median (Q1–Q3) | 2 (1–4) | 2 (1–4) | 0.497 c |
| Number of comorbidities for each patient, median (Q1–Q3) | 1(1–2) | 1(0–2) | 0.197 c |
| No. (%) of patients with hypertension | 86 (61) | 140 (49.6) | 0.027 a |
| No. (%) of patients with cardiovascular disease | 34 (24.1) | 90 (31.9) | 0.097 a |
| No. (%) of patients with diabetes | 37 (26.2) | 57 (20.2) | 0.160 a |
| No. (%) of patients with chronic obstructive pulmonary disease | 22 (15.6) | 27 (9.6) | 0.068 a |
| No. (%) of patients with chronic liver disease | 6 (4.3) | 8 (2.8) | 0.438 a |
| No. (%) of patients with chronic kidney disease | 16(11.3) | 22(7.8) | 0.229 a |
| No. (%) of patients with malignancy | 5 (3.5) | 13 (4.6) | 0.609 a |
| No. (%) of patients with dementia | 2 (1.4) | 13 (4.6) | 0.095 a |
Footnotes: a Chi-square test; b Student’s t-test; c Mann–Whitney test.
Table 2 compares the clinical and laboratory parameters of the patients in the Case and Control groups. The prevalence of pneumonia was very high in both groups (97.9% and 93.6%, respectively). Additionally, the clinical presentation at admission or in the last days before hospitalization was similar in the Cases and the Controls (Table 2), except for diarrhea, which was more frequent in the Case group (7.2% vs. 2.9%, p = 0.038), and asthenia, more frequent in the Control group (28.6% vs. 16.7%, p = 0.01) (Table 2). Moreover, the patients in the Case group showed at admission a lower value of PaO2/FiO2 ratio (P/F) (median 194 (Q1–Q3 119.5–300) vs. 232 (Q1–Q3 156–329), respectively; p = 0.014) (Table 2). Between the two groups, no statistical differences were observed at admission regarding laboratory parameters, except for blood creatinine level, which was more elevated in the obese group (median 0.9 mg/dL (Q1–Q3 0.8–1.1) vs. 0.84 mg/dL (Q1–Q3 0.7–1.025), respectively, p = 0.050) (Table 2).
Table 2.
Clinical and laboratory parameters at admission in patients in the Case and Control groups.
| Case Group | Control Group | p-Value | |
|---|---|---|---|
| No.of patients | 141 | 282 | |
| No. (%) of patients with fever | 87 (62.6) | 156 (55.5) | 0.167 a |
| No. (%) of patients with dyspnea | 103 (74.6) | 193 (68.7) | 0.208 a |
| No. (%) of patients with asthenia | 23 (16.7) | 79 (28.2) | 0.01 a |
| No. (%) of patients with cough | 45 (32.6) | 89 (31.8) | 0.865 a |
| No. (%) of patients with ageusia/dysgeusia | 4 (2.9) | 4 (1.4) | 0.302 a |
| No. (%) of patients with anosmia/hyposmia | 3 (2.2) | 6 (2.1) | 0.984 a |
| No. (%) of patients with diarrhea | 10 (7.2) | 8 (2.9) | 0.038 a |
| No. (%) of patients with skin lesions | 0 (0) | 0 (0) | - |
| Days from admission to discharge, median (Q1–Q3) | 14 (10–20) | 15 (10–21) | 0.555 c |
| No. (%) of patients with pneumonia | 138 (97.9) | 263 (93.6) | 0.057 a |
| Median (Q1–Q3) PaO2/FiO2 Ratio (P/F) | 194 (119.5–300) | 232 (156–329) | 0.014 c |
| Median (Q1–Q3) white blood cells | 7900 (5425–10355) | 7820 (5430–10730) | 0.858 c |
| Median (Q1–Q3) International Normalized Ratio (INR) | 1.11 (1.04–1.19) | 1.11 (1.04–1.21) | 0.775 c |
| Median (Q1–Q3) Blood creatinine | 0.9 (0.8–1.1) | 0.84 (0.7–1.025) | 0.050 c |
| Median (Q1–Q3) ALT | 36 (26–49) | 31 (21–51) | 0.130 c |
| Median (Q1–Q3) AST | 38 (26–58) | 33 (22–63.5) | 0.230 c |
| Median (Q1–Q3) total bilirubin | 0.6 (0.5–0.8) | 0.6 (0.41–0.85) | 0.946 c |
| Median (Q1–Q3) creatine phosphokinase (CPK) at admission | 99 (54.5–298) | 88 (57–166) | 0.347 c |
| Median (Q1–Q3) lacticodehydrogenase (LDH) | 323 (254–419) | 288 (229–411) | 0.099 c |
Footnotes: a Chi-square test; c Mann–Whitney test.
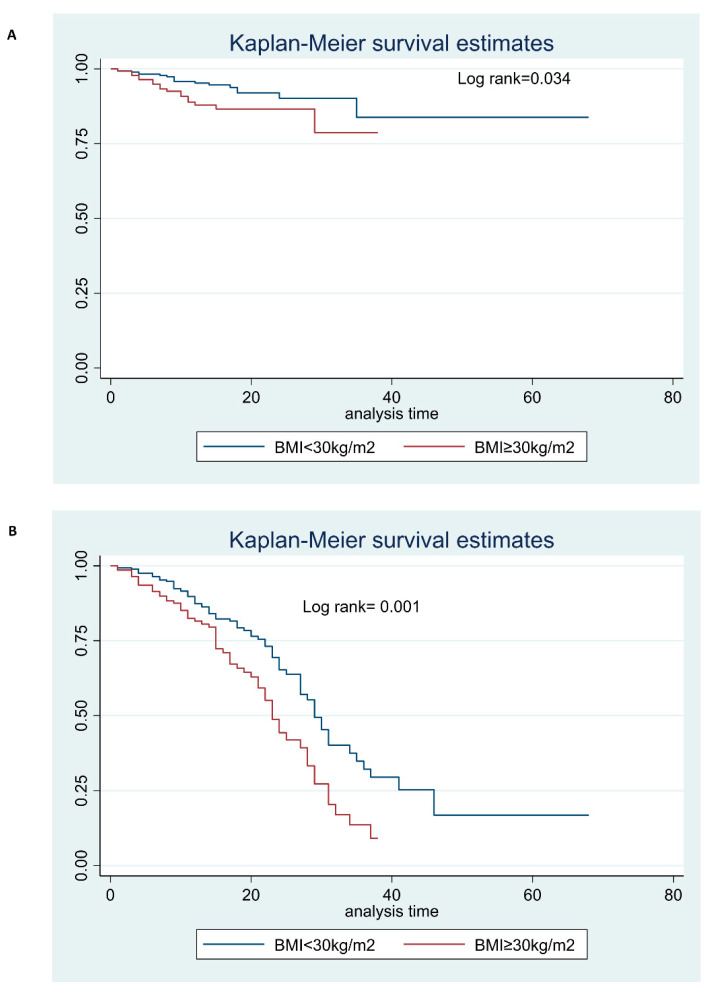
Table 3 shows the differences in the clinical outcome of the patients in the Case and Control groups. Thirty-five patients (8.3%) died during the hospitalization, all for respiratory failure due to COVID-19 pneumonia. Compared to the patients in the Case group, those in the Control group more frequently developed a mild–moderate disease (67% vs. 46.1%, respectively, p = 0.0001), whereas obese patients were more prone to be in need of intensive care treatment (41.8% vs. 26.6%, respectively; p = 0.001) (Table 3) (Figure 2A). Moreover, the prevalence of death during hospitalization was higher in the Case group than in the Control group (12.1% vs. 6.4%, p = 0.046) (Table 3) (Figure 2B). In conclusion, a severe outcome of COVID-19, with or without death during hospitalization was observed in 76 (53.9%) Cases and in 93 (33%, p < 0.0001) Controls.
Table 3.
Clinical outcome of patients in the Case and Control groups.
| Case Group | Control Group | p-Value | |
|---|---|---|---|
| No.of patients | 141 | 282 | |
| No. (%) of patients with mild or moderate outcome | 65(46.1) | 189(67) | 0.0001 a |
| No. (%) of patients needing intensive care treatment | 59 (41.8) | 75 (26.6) | 0.001 a |
| No. (%) of patients who died during hospitalization | 17 (12.1) | 18 (6.4) | 0.046 a |
Footnotes: a Chi-square test.
Figure 2.
(A) Survival curve estimate of patients stratified by BMI during hospitalization; (B) Survival curve estimate of ICU care of patients stratified by BMI during hospitalization.
4. Discussion
Obesity is one of the most important clinical conditions worldwide. The incidence of obesity in the general population is high and on the increase; worldwide, the prevalence rate for being overweight or obese between 1980 and 2013 increased to 27.5% for adults and 47.1% for children, i.e., a total of 2.1 billion individuals considered overweight or obese worldwide [20]. These increases were seen in both developed and developing countries. These data have an important impact on public health. In fact, for every 5-unit increase in BMI above 25 kg/m2, the overall mortality increases by 29%, vascular mortality by 41%, and diabetes-related mortality by 210% [20]. Excess energy from the diet is stored as fat in the white adipose tissue, distributed widely throughout the body and affecting whole-body homeostasis through metabolic, endocrine, and immune functions.
Data from the literature indicated that obese individuals were more susceptible to respiratory virus infection, and to SARS-CoV-2 infection, and showed a greater severity of illness and a worse outcome after infection, including higher rates of hospitalization, admission to ICU, and death [20]. However, obese subjects more frequently have other chronic conditions associated with a severe outcome of COVID-19 (CVDs, diabetes, liver, or kidney disease, etc.) [21].
Our case–control study investigated the role of obesity on the outcome of patients with SARS-CoV-2 infection, comparing the outcome of obese patients with those with BMI < 30 kg/m2, excluding other confounding factors. In fact, excluding gender, the patients with and without obesity were pair matched for the most important factors associated with the severity of COVID-19: age, CCI, and number of co-pathologies. Thus, although the Cases and Controls had similar age, CCI, and number of co-pathologies, the obese patients appeared more compromised than those with normal BMI at admission, showing lower PaO2/FiO2 ratio (p = 0.014), a parameter correlated with the efficiency of the respiratory function. Moreover, and more interestingly, the outcome of the obese patients was more severe: a higher percentage of patients needing intensive care (p < 0.001) or who died during hospitalization (p = 0.046) was observed in the obese patients than in those with BMI < 30 kg/m2.
Several previous studies evaluated this subject and demonstrated the association between overweight and severe outcome of COVID-19 [12,22,23,24,25], but often without considering other confounding factors. A Dutch study showed that 90% of the SARS-CoV-2-infected patients with respiratory failure had BMI higher than 25 kg/m2 and that the severity of the disease increased significantly with a high BMI [26]. Concurrently, COVID-19 patients requiring mechanical ventilation in a Seattle-based cohort had a mean BMI of 33 kg/m2 [22]. A southern California–based study linked BMI > 40 to greater relative risk among patients aged 60 years or younger and in men [23]. An international multicenter retrospective cohort study revealed a linear association between BMI and the need for invasive mechanical ventilation for critically ill COVID-19 patients, which was more pronounced in younger females and independent of other metabolic risk factors. In addition, a significant association between BMI and mortality was observed in obesity class III (≥40 kg/m2) [24].
There are several factors that may explain the association between obesity and severe outcome of COVID-19.
Obesity is associated with insulin resistance and over-activity of the renin–angiotensin–aldosterone system, which is related to worse outcomes in COVID-19 infection. ACE2, used by SARS-CoV-2 to infect cells, is more expressed in adipose tissue than in the lung. The obese population has more adipose tissue and consequently higher ACE2 levels that may enable the entry of SARS-CoV-2 into adipocytes, making adipose tissue an important viral reservoir able to spread the virus to other organs. Moreover, obesity is the leading cause of diabetes, which is also causally linked to elevated ACE2 expression. Cells expressing ACE2 are also connected to the progression of idiopathic pulmonary fibrosis. Adipocytes may substantially contribute to the production of circulating angiotensinogen, which after metabolism by renin and ACE produces angiotensin II (Ang II). Liu et al. observed that patients with COVID-19 infection were shown to have elevated Ang II levels that correlated with the severity of lung injury [12]. High Angiotensin II levels in the lung can induce pulmonary vasoconstriction, which leads to ventilation/perfusion mismatch and hypoxemia, as well as inflammation and oxidative damage, promoting acute lung injury [12].
The literature highlights that PaO2/FiO2 ratio at admission is a predictor of worse CT score in COVID-19 patients and a negative outcome of the disease [17]. Considering the lower PaO2/FiO2 ratio in patients with obesity compared with patients without, we underline that obesity is associated with impaired pulmonary function, with decreased expiratory reserve volume, functional capacity, and respiratory system compliance. Increased abdominal obesity compromises pulmonary function in supine patients by decreased diaphragmatic excursion, while the base of the lung ventilation is also impaired, resulting in reduced oxygen-saturated blood levels [20]. Furthermore, chronic low-grade inflammation and increased levels of circulating pro-inflammatory cytokines associated with obesity, such as leptin, tumor necrosis factor a, and interleukin 6, may impair immune response and affect the lung parenchyma [20].
The analysis in the present study showed that patients with diabetes had more frequent arterial hypertension (61% vs. 49.6%, p = 0.027). A recent meta-analysis on the impact of arterial hypertension on worse outcome in COVID-19 patients revealed a non-independent role in mortality [25]. Arterial hypertension is one of the comorbidities that, in association with others, define a patient that more frequently could have the worst outcome. Our data showed a high prevalence of arterial hypertension in obese patients, in line with studies published before, [9] but considering the setting of patients included in our study and the comorbidity associated with patients with obesity, compared to patients without, it is not clear if arterial hypertension is a leading actor or a spectator.
These data explain why obesity is considered one of the risk factors for the progression of COVID-19 infection and evolution of respiratory insufficiency and suggest that all patients with BMI > 30 should be considered to receive antivirals or monoclonal antibodies for COVID.
Our study shows several limitations. Firstly, its retrospective design, and secondly, not all patients included in the initial cohort had BMI data, even though the demographic and clinical data were similar in the subjects for whom the datum of BMI was available and in those for whom it was not, and we do not have BMI classes. Thirdly, no data were available on vitamin D’s role. Lastly, due to the historical period of our study, our cohort did not include subjects who were vaccinated against SARS-CoV-2 and/or with early treatments (antivirals or monoclonal antibodies), nor the impact of different variants. On the other hand, the strengths of our study may be the pair-match design, number of subjects enrolled, and multicenter design.
In conclusion, in our pair-matched 1:2 real-life study, we confirmed an association between obesity and the severe outcome of patients with COVID-19. In addition, patients with obesity showed a higher prevalence of arterial hypertension and a lower PaO2 FiO2 ratio at admission, but the impact of arterial hypertension in COVID-19 progression is still misunderstood. Thus, considering the pharmacological baggage we currently have and considering the relevant pathogenicity of the Omicron variant, and the results of our work, the BMI ≥ 30 kg/m2 is still a very important comorbidity in COVID-19 patients determining worse outcomes. Therefore, early therapy for SARS-COV-2 should be evaluated, whether antiviral or monoclonal antibodies, to avoid the development of severe disease.
Acknowledgments
Campania COVID-19 network: Nicola Coppola, Caterina Monari, Caterina Sagnelli, Paolo Maggi, Vincenzo Sangiovanni, Fabio Giuliano Numis, Ivan Gentile, Alfonso Masullo, Carolina Rescigno, Giosuele Calabria, Angelo Salomone Megna, Michele Gambardella, Elio Manzillo, Grazia Russo, Vincenzo Esposito, Giuseppina Dell’Aquila, Roberto Parrella, Rodolfo Punzi, Antonio Ponticiello, Mariantonietta Pisaturo, Enrico Allegorico, Raffaella Pisapia, Francesco Maria Fusco, Giovanni Porta, Margherita Macera, Federica Calò, Loredana Alessio, Cascone Angela, Caterina Monari, Onorato Lorenzo, Caterina Sagnelli, Stanzione Maria, Stornaiuolo Gianfranca, Anna Maria Rossomando, Mariana Di Lorenzo, Antonio Russo, Giorgio Bosso, Claudia Serra, Ferdinando Dello Vicario, Valentina Minerva, Giulia De Angelis, Stefania De Pascalis, Salvatore Martini, Paola Medusa, Ilaria Palma, Andrea Dell’Aquila, Nicola Carro, Roberta Astorri, Klodian Gjeloshi, Simona Imbriani, Carmen Ricozzi, Giovanni Di Caprio, Addolorata Masiello, Domenica Di Costanzo, Mariano Mazza, Vincenzo Bianco, Valeria Gentile, Antonio Riccardo Buonomo, Biagio Pinchera, and Riccardo Scotto.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12124055/s1, Table S1: STROBE Checklist.
Author Contributions
N.C., A.R. and M.P. were involved in study concept and design, drafting of the manuscript; S.M., P.M., I.G., F.G.N., R.P. (Roberto Parrella), G.C., E.M., A.M., G.D., G.R., M.G., A.P., R.P. (Raffaella Pisapia), V.Z., N.S., A.M.R., V.B., A.V.C. and L.O. were involved in critical revision of the manuscript for important intellectual content; S.M.; P.M., I.G., F.G.N., R.P. (Roberto Parrella), G.C., E.M., A.M., G.D., G.R., M.G., A.P., N.S., A.M.R., V.B., A.V.C. and R.P. (Raffaella Pisapia) and V.Z. were involved in acquisition of data, analysis, and interpretation of data and in critical revision of the manuscript; CoviCamp (Campania COVID-19 group) was involved in the enrolment of the patients. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agreed to be accountable for all aspects of the work. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee of the University of Campania L. Vanvitelli, Naples (No. 10877/2020, 11 May 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, N.C., upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
POR Campania FESR 2014–2020-Avviso per l’acquisizione di manifestazioni di interesse per la realizzazione di servizi di ricerca e sviluppo per la lotta contro il Covid-19 (DGR n. 140 del 17 marzo 2020), Regione Campania, Italy, Project: IDENTIFICAZIONE DEI FATTORI DEMOGRAFICI, CLINICI, VIROLOGICI, GENETICI, IMMUNOLOGICI E SIEROLOGICI ASSOCIATI AD OUTCOME SFAVOREVOLE NEI SOGGETTI CON COVID-19, and POR FESR Campania 2014–2020—Avviso per l’acquisizione di manifestazioni di interesse da parte degli Organismi di Ricerca per la realizzazione di servizi di ricerca, sviluppo e innovazione per la lotta contro il COVID-19 (DGR n. 504 del 10 November 2021)—Regione Campania, Italy; Project: IMPATTO DELLE NUOVE VARIANTI, L’USO DI TERAPIE ANTIVIRALI PRECOCI E STATO VACCINALE SULLA PRESENTAZIONE CLINICA DEL COVID-19: STUDIO RESTROSPETTIVO/PROSPETTICO MULTICENTRICO.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. WHO Coronavirus (COVID-19) Dashboard with Vaccination Data. [(accessed on 20 September 2022)]. Available online: https://covid19.who.int/
- 2.Macera M., De Angelis G., Sagnelli C., Coppola N. Vanvitelli COVID-19 group Clinical Presentation of COVID-19: Case Series and Review of the literature. Int. J. Environ. Res. Public Health. 2020;17:5062. doi: 10.3390/ijerph17145062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monari C., Sagnelli C., Maggi P., Sangiovanni V., Numis F.G., Gentile I., Masullo A., Rescigno C., Calabria G., Megna A.S., et al. More Severe COVID-19 in Patients with Active Cancer: Results of a Multicenter Cohort Study. Front. Oncol. 2021;11:662746. doi: 10.3389/fonc.2021.662746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pisaturo M., Calò F., Russo A., Camaioni C., Giaccone A., Pinchera B., Gentile I., Simeone F., Iodice A., Maggi P., et al. Dementia as Risk Factor for Severe Coronavirus Disease 2019: A Case-Control Study. Front. Aging Neurosci. 2021;13:698184. doi: 10.3389/fnagi.2021.698184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo A., Pisaturo M., Palladino R., Maggi P., Numis F.G., Gentile I., Sangiovanni V., Esposito V., Punzi R., Calabria G., et al. Prognostic Value of Transaminases and Bilirubin Levels at Admission to Hospital on Disease Progression and Mortality in Patients with COVID-19—An Observational Retrospective Study. Pathogens. 2022;11:652. doi: 10.3390/pathogens11060652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marin B.G., Aghagoli G., Lavine K., Yang L., Siff E.J., Chiang S.S., Salazar-Mather T.P., Dumenco L., Savaria M.C., Aung S.N., et al. Predictors of COVID-19 severity: A literature review. Rev. Med. Virol. 2021;31:1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milovanovic T., Pantic I., Dragasevic S., Lugonja S., Dumic I., Rajilic-Stojanovic M. The Interrelationship Among Non-Alcoholic Fatty Liver Disease, Colonic Diverticulosis and Metabolic Syndrome. J. Gastrointest. Liver Dis. 2021;30:274–282. doi: 10.15403/jgld-3308. [DOI] [PubMed] [Google Scholar]
- 8.Lopez-Jimenez F., Almahmeed W., Bays H., Cuevas A., Di Angelantonio E., le Roux C.W., Sattar N., Sun M.C., Wittert G., Pinto F.J., et al. Obesity and cardiovascular disease: Mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. Eur. J. Prev. Cardiol. 2022;29:2218–2237. doi: 10.1093/eurjpc/zwac187. [DOI] [PubMed] [Google Scholar]
- 9.Perone F., Pingitore A., Conte E., Halasz G., Ambrosetti M., Peruzzi M., Cavarretta E. Obesity and Cardiovascular Risk: Systematic Intervention Is the Key for Prevention. Healthcare. 2023;11:902. doi: 10.3390/healthcare11060902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell-Wiley T.M., Poirier P., Burke L.E., Després J.-P., Gordon-Larsen P., Lavie C.J., Lear S.A., Ndumele C.E., Neeland I.J., Sanders P., et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation. 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D’errico M., Pavlova M., Spandonaro F. The economic burden of obesity in Italy: A cost-of-illness study. Eur. J. Health Econ. 2022;23:177–192. doi: 10.1007/s10198-021-01358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou Y., Chi J., Lv W., Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (COVID-19) Diabetes/Metabolism Res. Rev. 2021;37:e3377. doi: 10.1002/dmrr.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fattori di Rischio Per la Salute: Fumo, Obesità, Alcol e Sedentarietà—Anno 2021. [(accessed on 20 September 2022)]. Available online: https://www.istat.it/it/archivio/270163.
- 14.Hill M.A., Sowers J.R., Mantzoros C.S. Commentary: COVID-19 and obesity pandemics converge into a syndemic requiring urgent and multidisciplinary action. Metabolism. 2021;114:154408. doi: 10.1016/j.metabol.2020.154408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalil A.C., Thomas P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care. 2019;23:258. doi: 10.1186/s13054-019-2539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pisaturo M., Russo A., Pattapola V., Astorri R., Maggi P., Numis F.G., Gentile I., Sangiovanni V., Rossomando A., Gentile V., et al. Clinical Characterization of the Three Waves of COVID-19 Occurring in Southern Italy: Results of a Multicenter Cohort Study. Int. J. Environ. Res. Public Health. 2022;19:16003. doi: 10.3390/ijerph192316003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo A., Pisaturo M., De Luca I., Schettino F., Maggi P., Numis F.G., Gentile I., Sangiovanni V., Rossomando A.M., Gentile V., et al. Lactate dehydrogenase and PaO2/FiO2 ratio at admission helps to predict CT score in patients with COVID-19: An observational study. J. Infect. Public Health. 2023;16:136–142. doi: 10.1016/j.jiph.2022.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calò F., Russo A., Palamone M., Maggi P., Allegorico E., Gentile I., Sangiovanni V., Russomando A., Gentile V., Calabria G., et al. Pre-existing chronic kidney disease (CDK) was not associated with a severe clinical outcome of hospitalized COVID-19: Results of a case-control study in Southern Italy. Infez. Med. 2022;30:539–546. doi: 10.53854/liim-3004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.StataCorp . Stata Statistical Software: Release 16. StataCorp LLC; College Station, TX, USA: 2019. [Google Scholar]
- 20.Apovian C.M. Obesity: Definition, comorbidities, causes, and burden. Am. J. Manag. Care. 2016;22((Suppl. 7)):s176–s185. [PubMed] [Google Scholar]
- 21.De Leeuw A.J.M., Oude Luttikhuis M.A.M., Wellen A.C., Müller C., Calkhoven C.F. Obesity and its impact on COVID-19. J. Mol. Med. 2021;99:899–915. doi: 10.1007/s00109-021-02072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatraju P.K., Ghassemieh B.J., Nichols M., Kim R., Jerome K.R., Nalla A.K., Greninger A.L., Pipavath S., Wurfel M.M., Evans L., et al. COVID-19 in critically ill patients in the seattle region—Case series. N. Engl. J. Med. 2020;382:2012–2022. doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tartof S.Y., Qian L., Hong V., Wei R., Nadjafi R.F., Fischer H., Li Z., Shaw S.F., Caparosa S.L., Nau C.L., et al. Obesity and mortality among patients diagnosed with COVID-19: Results from an integrated health care organization. Ann. Intern. Med. 2020;173:773–781. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., Wang Z., Li J., Li J., Feng C., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D’Elia L., Giaquinto A., Zarrella A.F., Rendina D., Idelson P.I., Strazzullo P., Galletti F. Hypertension and mortality in SARS-COV-2 infection: A meta-analysis of observational studies after 2 years of pandemic. Eur. J. Intern. Med. 2023;108:28–36. doi: 10.1016/j.ejim.2022.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Voort P.H.J., Moser J., Zandstra D.F., Muller Kobold A.C., Knoester M., Calkhoven C.F., Hamming I., van Meurs M. Leptin levels in SARS-CoV-2 infection related respiratory failure:a cross-sectional study and a pathophysiological framework on therole of fat tissue. Heliyon. 2020;6:e04696. doi: 10.1016/j.heliyon.2020.e04696. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, N.C., upon reasonable request.