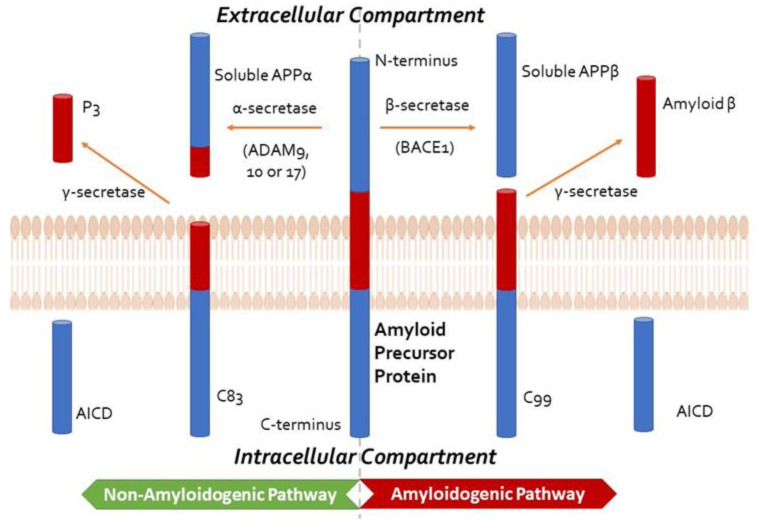
Figure 1.
Summary of Amyloid Precursor Protein (APP) processing, depicting the non-amyloidogenic and the amyloidogenic pathways, the former precluding the formation of amyloid-β. In the non-amyloidogenic pathway, APP is cleaved by an α-secretase from the A disintegrin and metalloprotease (ADAM) family, such as ADAM9, ADAM10, and ADAM17, to form soluble APPα and C83. The smaller carboxy-terminal fragment, C83, can be cleaved by γ-secretase to generate P3 and the APP intracellular domain (AICD, not shown). In the amyloidogenic pathway, APP is cleaved by β-secretase (β-site APP-cleaving enzyme 1, BACE1) to produce soluble APPβ, retaining the last 99 amino acids of APP (known as C99) within the membrane. The peptide C99 is then cleaved by the γ-secretase complex, comprising presenilin 1 or 2, nicastrin, anterior pharynx defective-1 (APH-1), and presenilin enhancer 2 (PEN2) at the amino terminus to form amyloid-β and AICD. This cleavage predominantly produces Aβ1-40, and the more amyloidogenic Aβ1-42 at a ratio of 10:1.