Figure 1. Pathological ECM remodelling in obesity.
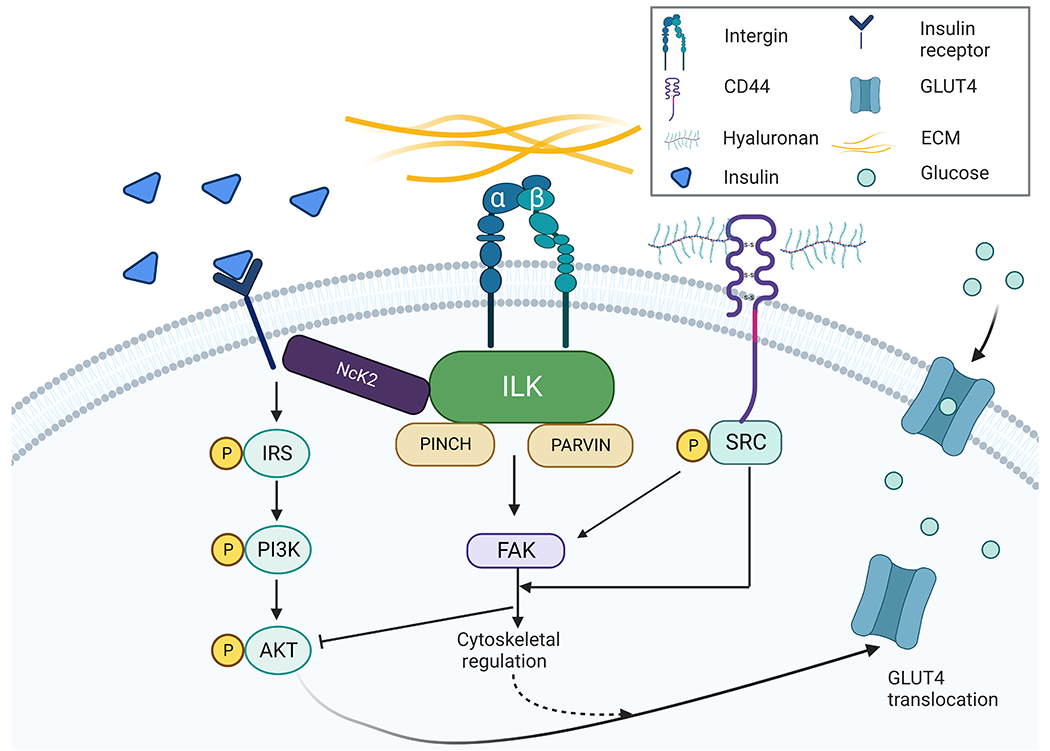
Obesity induces a maladaptive ECM remodelling by increasing deposition of its components and activating downstream ECM receptor signalling (e.g. integrin and CD44). ILK, a primary modulator of integrin signalling, interacts with the cytoplasmic domain of integrins and forms an ILK-PINCH-Parvin (IPP) protein complex, which recruits adaptor proteins such as Nck2 to interact with tyrosine kinase receptors including the insulin receptor. Under obese condition, overactivation of CD44 signalling has been shown to suppress AKT phosphorylation. In response to excessive ECM deposition in obese state, integrin and CD44 signalling cascades have been linked to impaired GLUT4 translocation and glucose transport under insulin stimulation in fat and muscle cells.
