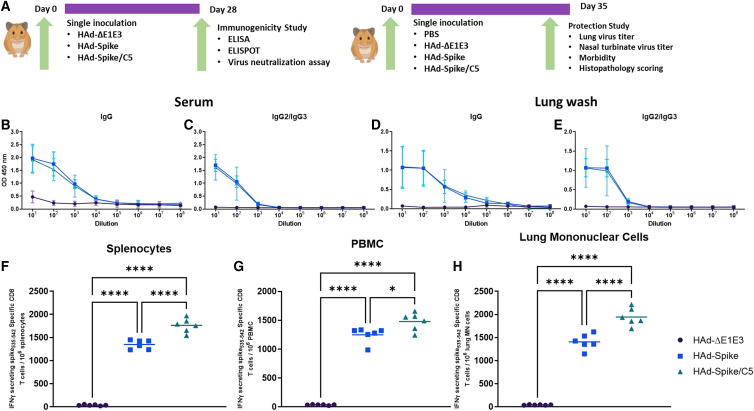
Figure 4.
Study design and immunogenicity of HAd-Spike and HAd-Spike/C5 in golden Syrian hamsters
(A) Outlines of the 1D immunogenicity study (right panel) and protection study against SARS-CoV-2 (B.1.1.28) challenge (left panel) following a 1D regimen in the golden Syrian hamster model. Six- to seven-week-old golden Syrian hamsters (3 males and 3 females/group) were inoculated i.n. once with 2 × 108 PFUs/animal of HAd-ΔE1E3, HAd-Spike, or HAd-Spike/C5. (B and C) Four weeks post immunization, blood samples were collected and used to study the development of Spike-specific IgG (B) and IgG2/IgG3 (C) antibody responses by ELISA. (D and E) Similarly, lung washes were used for monitoring the development of Spike-specific IgG (D) and IgG2/IgG3 (E) antibody responses by ELISA. ELISA data are shown as the mean ± SD of the OD readings with log10 diluted samples. (F–H) The development of Spike-specific T cell response after vaccination was investigated in splenocytes (F), peripheral blood MN cells (PBMCs) (G), and lung MN cells (H) by counting Spike-specific IFN-γ-secreting CD8 T cells by ELISpot. ∗p ≤ 0.05, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001.