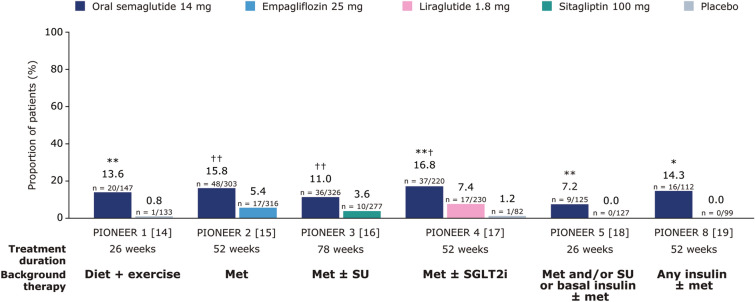
Fig. 3.
Proportion of people achieving an HbA1c reduction of ≥ 1% and body weight loss of ≥ 10% at EOT. Data are observed proportions for the trial product estimand (on trial product without rescue medication). For PIONEER 1–4, p values are for the EORs for orally administered semaglutide 14 mg versus placebo or the active comparator. For PIONEER 5 and 8, EORs could not be calculated as a result of 0 events in the comparator arms; p values for the risk difference were calculated instead. *p < 0.05 versus placebo; **p < 0.01 versus placebo; ***p < 0.001 versus placebo; †p < 0.001 versus the active comparator; ††p < 0.0001 versus the active comparator. EOR estimated odds ratio, EOT end of treatment, HbA1c glycated haemoglobin, met metformin, SGLT2i sodium-glucose co-transporter 2 inhibitor, SU sulphonylurea