FIGURE 4.
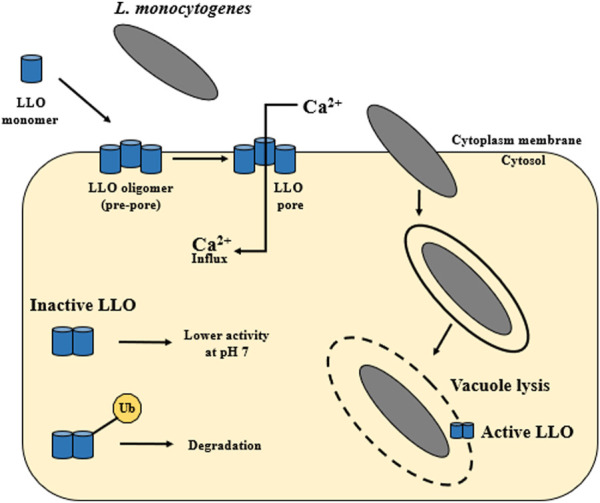
The activity of listeriolysin O. LLO is secreted as a water-soluble monomer that binds to cholesterol in host membranes. Then it oligomerizes into large complex—a toxin is formed, which can generate pores. The main function of LLO is the release of L. monocytogenes from the vacuole into the host cytoplasm. Additionally, the activity of LLO is pH dependent. Listeriolysin O has the so-called pH sensor. The highest activity of LLO is observed at the acidic pH of the vacuole (pH 5.5), while at the neutral pH of the host cytoplasm this activity decreases. Cytosolically synthesized LLO is first ubiquitylated and then LLO monomers are degraded by the proteasome. LLO participates in membrane internalization through membrane binding (cholesterol-rich) and oligomerization (pre-pore complex). This complex passes into transmembrane pores that allow the influx of extracellular Ca2+ (according to: Vadia et al., 2011; Arnett et al., 2014; Osborne and Brumell, 2017; Chen et al., 2018; Lam et al., 2018).
