Abstract
The importance of fluid resuscitation therapy during the early stages of sepsis management is a well-established principle. Current Surviving Sepsis Campaign (SSC) guidelines recommend the early administration of intravenous crystalloid fluids for sepsis-related hypotension or hyperlactatemia due to tissue hypoperfusion, within the first 3 h of resuscitation and suggest using balanced solutions (BSs) instead of normal saline (NS) for the management of patients with sepsis or septic shock. Studies comparing BS versus NS administration in septic patients have demonstrated that BSs are associated with better outcomes including decreased mortality. After initial resuscitation, fluid administration has to be judicious in order to avoid fluid overload, which has been associated with increased mortality, prolonged mechanical ventilation, and worsening of acute kidney injury. The “one size fits all” approach may be “convenient” but it should be avoided. Personalized fluid management, based on patient-specific hemodynamic indices, provides the foundations for better patient outcomes in the future. Although there is a consensus on the need for adequate fluid therapy in sepsis, the type, the amount of administered fluids, and the ideal fluid resuscitation strategy remain elusive. Well-designed large randomized controlled trials are certainly needed to compare fluid choices specifically in the septic patient, as there is currently limited evidence of low quality. This review aims to summarize the physiologic principles and current scientific evidence regarding fluid management in patients with sepsis, as well as to provide a comprehensive overview of the latest data on the optimal fluid administration strategy in sepsis.
Keywords: fluids, resuscitation, sepsis, septic shock, balanced crystalloids, saline, liberal fluids, restricted fluids
1. Introduction
Sepsis is defined as a life-threatening organ dysfunction caused by a dysregulated host response to infection, whereas septic shock is a subset of sepsis in which profound circulatory, cellular, and metabolic abnormalities are associated with a higher risk of mortality than with sepsis alone [1]. Although the exact worldwide burden of sepsis is difficult to ascertain, it certainly represents a major global health issue. In 2017, there was an estimate of 48.9 million cases of sepsis; during the same year, 11 million sepsis-related deaths were reported worldwide, representing almost 20% of global deaths [2]. Between 1990 and 2017, age-standardized sepsis incidence fell by 37% and mortality decreased by 52.8% [2]. Despite these trends, sepsis still remains a major cause of death worldwide. Interestingly, there are significant regional disparities in sepsis-related incidence and mortality, with approximately 85% of sepsis cases and sepsis-related deaths occurring in low- and middle-income countries [2].
The management of sepsis has not significantly changed over the past 40 years. Current guidelines recommend the early administration of antibiotics and intravenous (IV) fluids, in addition to source control and the judicious use of vasopressors [3]. Fluid resuscitation therapy represents one of the cornerstones of sepsis management [3]. Understanding the pathophysiology of sepsis is crucial in order to determine the role of intensive fluid administration in the initial phase of septic shock.
Although there is a consensus on the need for adequate fluid therapy in sepsis and despite the multiple recent clinical trials examining fluid management in sepsis, the ideal fluid management strategy is still controversial and elusive, as there are no clear guidelines about the optimal fluid resuscitation in critically ill patients with sepsis. The purpose of this narrative review is to summarize the physiologic principles and current scientific evidence regarding fluid management in patients with sepsis, as well as to provide a comprehensive overview of the latest data on the optimal type (balanced crystalloids versus normal saline) and volume (liberal versus restricted administration) of fluids in sepsis and septic shock patients.
2. Methods
A literature search was performed to identify all published research, such as original articles, reviews, and systematic reviews/metanalyses, using the key words “fluid resuscitation”, “sepsis”, “septic shock”, “critically-ill”, balanced crystalloids”, “normal saline”, “liberal fluid administration”, and “restricted fluid administration”. Records were retrieved from PubMed/Medline and Scopus, without prior application of language or other restrictions for the database search. Reference lists of included articles were also screened to identify potential studies missed by the initial literature search.The physiology of fluid administration in sepsis is a complex syndrome with multiple underlying mechanisms contributing to its pathogenesis. The initial step involves the recognition and the binding of pathogen-associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) on the surface of host immune cells [4,5]. Toll-like receptors (TLRs) are a crucial group of PRRs that play a significant role in initiating the immune response [4,5]. They recognize various PAMPs such as bacterial lipopolysaccharide, viral RNA, and fungal cell wall components [4,5]. PRRs can also recognize endogenous danger-associated molecular patterns (DAMPs) that are released during the inflammatory insult [4,5]. Upon pathogen recognition, immune cells release pro-inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) [4,6]. These cytokines trigger a cascade of immune responses and recruit additional immune cells to the site of infection, such as polymorphonuclear leukocytes and macrophages [4,6]. In sepsis, the immune response becomes dysregulated, leading to an excessive and uncontrolled release of pro-inflammatory cytokines, commonly referred to as a cytokine storm. This hyperinflammatory state contributes to tissue damage and organ dysfunction [4,6]. Simultaneously, there is an activation of the anti-inflammatory response, resulting in immunosuppression by inhibiting cytokine production by mononuclear cells and monocyte-dependent T helper cells [4,6].
Sepsis is characterized by a dysregulated inflammatory response with derangement of both macro- and microcirculation, leading to a status of actual and relative hypovolemia, resulting in tissue hypoperfusion and imbalance between oxygen delivery and demand [7,8]. The absolute and relative intravascular volume depletion in septic patients is attributed to gastrointestinal fluid losses, insensible loss from tachypnea, anorexia with decreased oral intake, arterial vasodilation, cytokine-mediated injury of the endothelium leading to capillary leaks and fluid extravasation to the interstitial compartment, and venodilation with increased venous capacitance [9,10,11]. All these mechanisms can be present in varying degrees among patients and are responsible for the reduction in stressed volume, venous return, and therefore, ventricular preload and cardiac output, which further promotes tissue hypoxia [11,12].
Fluid administration is considered the cornerstone of initial hemodynamic resuscitation in sepsis in order to restore circulating fluid volume and increase cardiac output and eventually, oxygen delivery [13,14]. Fluid resuscitation exerts its therapeutic effect if the augmentation of stressed volume results in a pressure gradient for venous return that exceeds the central venous pressure (CVP) [14]. The subsequent increase in cardiac output restores the mean arterial pressure (MAP) and thus, improves microcirculatory flow and perfusion pressure, reducing the risk of tissue hypoperfusion and subsequent ischemic damage [15]. This concept is consistent with the Frank–Starling principle which demonstrates that under normal physiological circumstances, increasing the preload could optimize stroke volume, even though under a septic state, this therapeutic benefit may be affected [11,16]. The restoration of intravascular volume supports the renal function, increasing diuresis and the clearance of metabolic waste products [17]. Furthermore, fluid resuscitation contributes to the stabilization of electrolyte and acid–base balance, resulting in the maintenance of cellular homeostasis [17]. Fluid resuscitation also aims to maintain the microvascular integrity and endothelial function, preventing endothelial barrier dysfunction and reducing tissue edema [18]. These effects are essential in promoting oxygen and nutrient delivery to the tissues and supporting organ function during critical illness and sepsis [18].
This reasonable and well-established mechanism is excessively simplistic and it is increasingly recognized that, apart from hemodynamics, oxygen delivery and organ perfusion are also affected by the bioenergetic failure and impaired oxidative metabolism [19,20]. This is best demonstrated in the septic heart, where aggressive volume resuscitation may paradoxically result in cardiovascular collapse and impaired myocardial contractility. This can be explained by mitochondrial oxidative stress, microvascular thrombosis, and increased myocardial edema [21,22,23]. Indeed, despite an apparent initial improvement after fluid administration, eventually, half of septic patients become non-responsive to fluids, experiencing significant harmful effects such as fluid extravasation, decreased venous return, and impaired tissue perfusion, with a minimal increase in end-diastolic volume [24,25]. In addition, while the purpose of fluid resuscitation is the improvement of hemodynamic parameters with CVP > 8 mmHg and MAP > 65 mmHg, a compensatory decrease in systemic vascular resistance (SVR) may occur, worsening the clinical outcomes [19,26]. This has been attributed to the inhibition of sympathetic activity and the augmentation of endothelial shear stress in response to fluid administration, which are followed by an increase in nitric oxide release and, ultimately, vasodilation [27,28,29]. Fluid bolus administration may result in a decrease in SVR through hemodilution and reduced blood viscosity [15].
Excessive fluid resuscitation may exacerbate shock, as a significant increase in filling pressures can overcome cardiac compensation mechanisms when the patient reaches the plateau of the Frank–Starling curve [19,30,31]. The subsequent high left atrial pressure leads to pulmonary congestion and edema, the potential development of pulmonary hypertension, and finally, left ventricle dysfunction and a reduction in left-ventricular volume and cardiac output [32,33]. Similarly, high right atrial pressure results in a decrease in venous return and retrograde increased venous pressure. This results in fluid extravasation into the interstitial space and subsequent tissue edema, which leads to architecture distortion and increased resistance to capillary blood flow and lymphatic drainage [13,14,28].
Moreover, sepsis produces alterations in vascular permeability and only 5% of fluid bolus volume remains intravascular after 90 min in the critically ill patient, due to rapid fluid redistribution [30]. It is well established that sepsis leads to damage of the endothelial glycocalyx, which is a main determinant of membrane permeability and vascular homeostasis [13,34,35]. Glycocalyx degradation may be exacerbated due to fluid overload, as the increase in cardiac filling pressures results in the release of natriuretic peptides that cleave proteoglycans and glycoproteins from the glycocalyx [36,37]. Consequently, the high capillary leak translates clinically into inadequate and short-lived responses of hemodynamic parameters to fluid administration with increased tissue edema, decreased oxygen diffusion, and possible organ failure [14,38,39]. The better understanding of the complexity of septic patient response to fluid resuscitation, especially after the recent recognition of the role of endothelial glycocalyx, has led to the development of a revised Starling principle [9,20]. This new model demonstrates the important role of glycocalyx for transvascular fluid exchange, allowing for more efficient therapeutic strategies to be designed in order to improve patient outcomes [40].
The volume and the type of fluid used for initial resuscitation and the maintenance of fluid therapy have an impact on salt and water retention and by extension to fluid overload, which affects all the major organ systems, causing potential unfavorable outcomes on end-organ function [41,42]. More specifically, aggressive fluid resuscitation may result in secondary intra-abdominal hypertension, which is associated with acute kidney injury, hepatic venous congestion, respiratory dysfunction with pulmonary edema, as well as disorders in the central nervous system, such as cerebral edema and intracranial hypertension and in the cardiovascular system, such as myocardial edema, decreased ejection fraction, and cardiac output, leading to multi-organ failure and death [19,43,44].
3. Fluid Resuscitation in Sepsis
Despite the scientific advances of the last 20 years, sepsis management has not changed drastically, apart from the introduction of the bundles, which designate multiple interventions that should be completed within a specific time frame. After initial airway and respiratory stabilization, sepsis bundle should be performed within the first 3 h of presentation. The SSC 2021 bundle includes fluid resuscitation, antibiotic administration, lactate measurement and obtainment of cultures [3]. Vasopressors should be initiated if the patient remains hypotensive despite adequate fluid resuscitation [3]. However, a group of 34 European Society of Intensive Care Medicine (ESICM) experts recently suggested to start vasopressors early, before full completion of fluid resuscitation [45]. In the revision of the Surviving Sepsis Campaign (SSC) guidelines in 2018, the 3 and 6 h bundles were combined into a single “1-h bundle” where fluid resuscitation is required in all patients without exception [46]. The implementation of these sepsis protocols in clinical practice have led to decreased sepsis mortality [47].
Fluid resuscitation remains an integral part of sepsis management, since it was first employed during the European cholera epidemic as early as 1830 [48]. The following years, fluid resuscitation was used to treat hypovolemia and restore tissue perfusion pressure in order to improve oxygen transport to cells [49]. Previous versions of SSC guidelines recommended a quantitative resuscitation protocol, that was based entirely on the early goal-directed therapy (EGDT) study [50]. This landmark study showed the benefit of early and aggressive fluid resuscitation in the mortality and the maintenance of a CVP of 8–12 mmHg and a central venous oxygen saturation (SCVO2) of at least 70% [50]. The era of a time-sensitive bundled care was then introduced in sepsis. However, subsequent multi-center randomized controlled trials (RCTs) failed to reproduce the benefits observed in the EGDT trial [51].
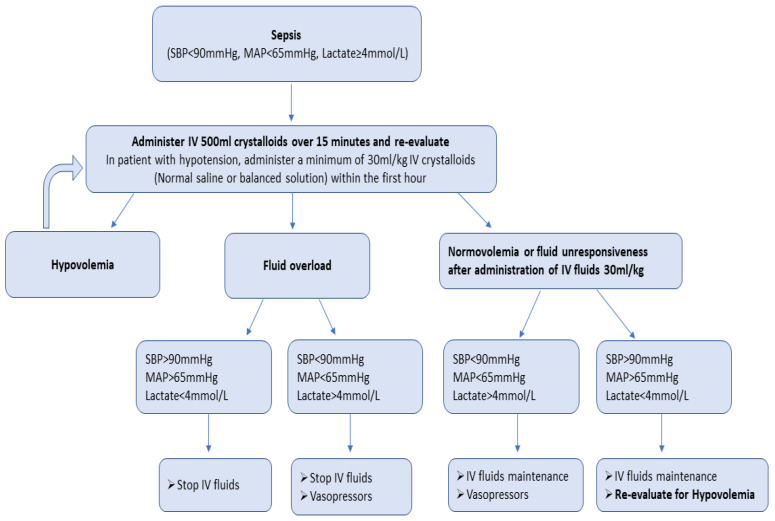
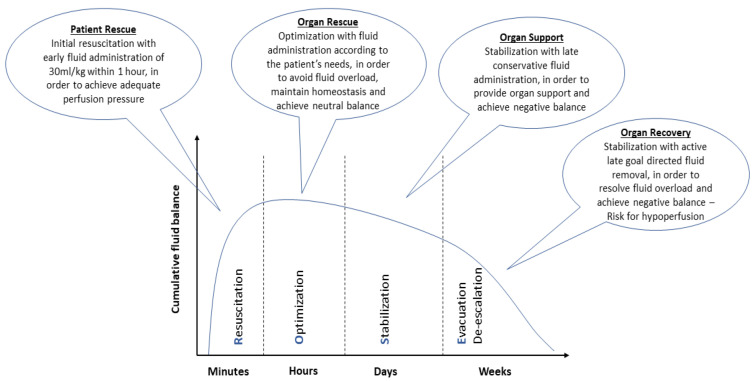
There is a growing scepticism regarding aggressive fluid resuscitation, since this approach may lead to massive fluid overload and, inevitably, to adverse outcomes [52]. An increasing number of studies have associated fluid overload to worse outcomes and increased mortality in septic patients [31,33,53]. Current SSC guidelines recommend the early administration of 30 mL/kg of IV fluids for sepsis-related hypotension or a lactate ≥ 4 mmol/L, within the first 3 h of resuscitation [3]. This recommendation remains weak, as it is based on low-quality evidence. Infusing an initial 1 L bolus over the first 30 min and administrating the remainder volume of fluid resuscitation with repeated bolus infusions is an acceptable approach [54]. A proposed algorithm about fluid resuscitation in patients with sepsis is shown in Figure 1 [3,8,10]. Four distinct phases of IV fluid therapy have been proposed: resuscitation, optimization, stabilization, and evacuation (ROSE), which are all crucial steps in sepsis management (Figure 2) [55]. In addition, specific strategies for fluid minimization and de-escalation or de-resuscitation have been reported, demonstrating that fluid restriction is associated with improved outcomes [30,56].
Figure 1.
Proposed algorithm of fluid resuscitation in patients with sepsis.
Figure 2.
Characteristics of the four distinct phases of intravenous fluid therapy: resuscitation, optimization, stabilization, and evacuation (ROSE).
The 2021 SSC guidelines suggest the use of crystalloid fluids [3]. However, different types of fluids have been proposed. Colloids, including albumin and semisynthetic colloids, such as hydroxyethyl starch (HES), dextrans, and gelatins, were commonly used in the past. Several studies which examined their use in septic patients recommend against the administration of HES and other semisynthetic colloids [57,58,59,60,61]. HES use has been associated with acute kidney injury and the need for renal replacement therapy, as well as with increased mortality [61]. Gelatins have been found to increase anaphylaxis, renal failure, bleeding, and mortality [62]. Hence, the side effects of semisynthetic colloids far outweigh any potential benefits and, according to the SSC guidelines, their use should be avoided in sepsis management [3].
Current SSC guidelines suggest using albumin in septic patients who received large volumes of crystalloids over using crystalloids alone [3]. Albumin is not recommended as the first-line fluid for resuscitation in sepsis due to the lack of proven benefit and its higher cost compared to crystalloids [3]. However, two RCTs, the Saline versus Albumin Fluid Evaluation (SAFE) and the Albumin Italian Outcome Sepsis (ALBIOS) study, as well as a meta-analysis of randomized clinical trials, compared the effect of albumin and crystalloid use in patients with sepsis or septic shock, and showed a trend towards reduced mortality and improved outcomes in the albumin group, without observing serious side effects [63,64,65].
In septic patients, human albumin solution can be given for two indications: to restore or expand intravascular volume and to supplement serum albumin in the septic patients with hypoalbuminemia [66]. In addition, human albumin acts as the most significant modulator of plasma oncotic pressure, which is typically in the 25–30 mmHg range. This is a major endogenous antioxidant agent and a major binding protein of several endogenous compounds and drugs [66]. Albumin appears to have important immunomodulatory effects that likely impact the host inflammatory response in critical illness [66]. The time, dose, and concentration of the albumin, as well as the determination of a specific target for serum albumin level remains controversial. Of note, in the ALBIOS trial, albumin was administered as a 20% solution, with a treatment goal of a serum albumin concentration of 30 g/L until intensive care unit (ICU) discharge or 28 days [64].
4. Balanced Crystalloids versus Normal Saline in Sepsis and Septic Shock
The ideal fluid for septic patients, which would have similar osmolarity to plasma, increase intravascular volume and cardiac output, and improve tissue perfusion without causing tissue edema, while at the same time be cost effective, has yet to be discovered [9].
Fluids are classified according to their composition in two major categories: crystalloid and colloid solutions. Crystalloids are recommended as first-line resuscitation fluids in patients with sepsis as they are inexpensive, widely available, and lead to fewer serious adverse effects [67,68]. Isotonic crystalloids have a tonicity similar to plasma and are further divided into balanced and unbalanced solutions. Herein, we summarize the current evidence regarding the use of the two different types of crystalloids in patients with sepsis and septic shock, and their effects on patient outcomes (Table 1).
Table 1.
Characteristics of the key randomized controlled trials assessing balanced solutions versus normal saline in patients with sepsis and septic shock.
| Balanced Solutions versus Normal Saline in Sepsis | ||||||
|---|---|---|---|---|---|---|
| Study ID | Year | Sample Size | Population | Intervention | Comparison | Outcome |
|
SPLIT [64] |
2015 | 2262 | ICU patients | Plasma-Lyte148 (median volume 2000 mL) N = 1152 |
NS (median volume 2000 mL) N = 1110 |
No significant difference in the AKI and mortality within 90 days. |
|
SALT [65] |
2017 | 974 | ICU adult patients | BS (median volume 1617 mL) N = 520 |
NS (median volume 1424 mL) N = 454 |
No significant difference in the AKI and mortality within 30 days. More major kidney events in the NS group. |
|
SMART [66] |
2019 | 1641 | ICU adult patients | BS Plasma-Lyte A and Lactated Ringer’s (mean volume 2967 mL) N = 824 |
NS (mean volume 3454 mL) N = 817 |
Lower incidence of mortality and major adverse kidney events within 30 days in the BS group. Greater number of vasopressor-free days and renal replacement therapy-free days in the BS group. |
|
BaSICS [69,70] |
2021 | 10,520 | ICU adult patients | BS Plasma-Lyte (median volume 1500 mL) N = 5230 |
NS (median volume 1500 mL) N = 5290 |
No significant difference in the AKI and mortality within 90 days. Higher 90-day survival in the subgroup of septic patients receiving balanced crystalloids. |
|
PLUS [71] |
2022 | 5037 | ICU adult patients | Plasma-Lyte 148 (median volume 3900 mL) N = 2515 |
NS (median volume 3700 mL) N = 2522 |
No significant difference in the AKI and mortality within 90 days. |
| PRoMPTBOLUS [72] | Ongoing | Estimated size: 8800 | Pediatric patients with sepsis | BS | NS | In progress |
AKI: acute kidney injury; BS: balanced solution; ICU: intensive care unit; MV: mechanical ventilation; NS: normal saline.
4.1. Unbalanced Solutions
The most commonly used unbalanced solution is 0.9% normal saline (NS). It is an isotonic solution and contains equal concentrations of sodium and chloride (154 mmol/L) and no organic anion to act as acid buffer. As a result, it has a strong ion difference equal to zero. Notably, its chloride concentration is almost 40% higher than that of plasma. This high chloride concentration has been associated with hyperchloremic metabolic acidosis, impaired tissue and renal perfusion, acute kidney injury, coagulopathy, and altered inflammatory response [69,70,71,73,74].
4.2. Balanced Solutions
Balanced solutions (BSs), apart from sodium and chloride, include other ions, such as potassium, calcium, and magnesium, and may also contain buffers such as bicarbonate, lactate, acetate, or gluconate, leading to electroneutrality (balance between positive and negative anions) [75]. Moreover, a crystalloid solution is considered balanced when it has a strong ion difference close to 24 mEq/L and contains chloride similar to plasma’s chloride concentration (98–112 mmol/L) [76]. Commonly used BSs are lactated Ringer’s, Hartmann’s solution, Plasma-Lyte, and Normosol. More specifically, lactated Ringer’s solution consists of sodium, chloride, potassium, calcium, and sodium lactate mixed into a solution with an osmolality of 273 mOsm/L and pH of 6.5 [77]. Hartmann’s solution is similar to lactated Ringer’s, while the main difference of Plasma-Lyte is that it does not contain calcium [78]. Finally, Normosol, like Plasma-Lyte, is calcium-free solution and is composed of sodium, potassium, magnesium, chloride, acetate, and gluconate, while its pH is 7.4 [79].
4.3. Studies Comparing BSs versus NS in Sepsis and Septic Shock
Numerous studies have been carried out in order to investigate and compare the effectiveness of BSs and NS on septic patients’ outcomes and identify the optimal fluid solution. However, which type of crystalloid solution should be administered during the management of patients with sepsis and septic shock remains unanswered, due to the low quality of existing evidence. Current SSC guidelines strongly recommend crystalloids as first-line fluid resuscitation, and further suggest using a BS instead of NS for the management of patients with sepsis or septic shock (weak recommendation, low quality evidence) [3].
A double-blind, cluster randomized, double-crossover trial was conducted in four ICUs in New Zealand during a 7-month period (the Saline vs. Plasma-Lyte 148 for ICU fluid Therapy (SPLIT) trial) aiming to determine the effect of BS in comparison with NS on acute kidney injury [80]. The results of this study did not reveal significant differences in the outcomes of incidence of acute kidney injury or mortality among critically ill patients who received BS or NS; however, among the enrolled patients, only 4% were septic [80]. On the other hand, another cluster-randomized, multiple cross-over trial (the isotonic Solution Administration Logistical Testing (SALT) trial) compared the impact of BSs versus NS on patient outcomes in ICU and showed that patients who received larger volumes of NS appeared to experience more frequent major renal complications; the proportion of septic patients who received a BS and NS was 28.6% and 25%, respectively [81].
In 2018, the single-center cluster-randomized isotonic Solutions and Major Adverse Renal Events Trial (SMART) was conducted in five ICUs and compared the effect of BS and NS on mortality and renal outcomes [82]. This was the first RCT, demonstrating that the IV administration of BS during fluid resuscitation was associated with lower mortality and more favorable outcomes regarding the need for renal replacement therapy and persistent renal dysfunction, compared to NS [82]. More specifically, a secondary analysis of the SMART trial which focused on critically ill adults with sepsis revealed that the use of BSs was associated with a lower 30-day in-hospital mortality compared to NS [83]. Additionally, an ancillary analysis of the SMART trial demonstrated that the use of BSs was associated with a modest decline in early biomarkers of acute kidney injury [72].
Conversely, the large RCT Balanced Solution in Intensive Care Study (BaSICS), which aimed to determine the effect of BS (Plasma-Lyte 148) or NS on 90-day survival in ICU patients, did not reveal any significant reduction in mortality between the two groups [84]. Interestingly, a secondary post hoc analysis demonstrated that, especially in the subgroup of septic patients who exclusively received BS before trial enrolment, the probability of 90-days survival was higher. As a result, the type of fluid used for the initial resuscitation may alter the outcomes in septic shock patients, as these patients seem to be more sensitive to external chloride overload possibly due to the decreased albumin synthesis caused by the inflammatory response [85].
Recently, another RCT, the Plasma-Lyte 148 versus Saline Study (PLUS) trial examined the relationship between the administration multi-electrolyte BS (Plasma-Lyte 148) versus NS, and the outcomes of 90-day mortality and renal complications in critically ill adults [86]. Notably, 42.3% of the enrolled patients had sepsis. In contrast to other trials, the PLUS trial did not find any significant difference between the two types of fluids [86].
Multiple observational, retrospective, and cohort studies have also been published, comparing the use of BS and NS. Specifically, a retrospective cohort study was designed to determine the effect of a BS (Normosol) compared with NS on the outcomes of patients with sepsis, defined as acute kidney injury and the need for renal replacement therapy [79]. This study did not reveal any difference among septic patients who were resuscitated with either Normosol or NS [79]. Similarly, no statistically significant difference was observed among septic patients treated with lactated Ringer’s solution or NS regarding serum lactate clearance, serum creatinine change within 24 h, and 48 h survival after admission at the emergency department [87]. On the contrary, other studies support the use of BS over NS in septic shock patients, as NS might be associated with renal complications, increased bleeding risk, and mortality [68,88,89,90].
Various systematic reviews and meta-analyses have been conducted comparing the two fluid treatments. Of note, the vast majority of these studies did not reveal any significant difference in the effect of BS or NS on the outcomes of patients with sepsis [91,92,93,94]. However, the most recent meta-analysis demonstrated that BS administration in septic patients was associated with decreased mortality and acute kidney injury as compared with NS, although subgroup analysis including only RCTs did not show any difference between the two groups [95].
Despite the abundance of data regarding the effectiveness and safety of BS and NS use on adults with sepsis, few studies have been carried out in children. Currently, NS remains the preferred resuscitation fluid for children with sepsis, although the SSC guidelines suggest the use of BS rather than NS for the initial resuscitation (weak recommendation, very low quality of evidence) [96]. Due to the scarcity of evidence to support BS or NS, a large Pragmatic Pediatric trial of Balanced versus NS Fluid in sepsis (PRoMPT BOLUS) is now being conducted in order to establish clear, high-quality evidence and determine whether the use of BS in pediatric patients with sepsis is associated with improved outcomes compared to NS [97].
5. Liberal versus Restricted Fluid Administration in Sepsis and Septic Shock
Fluid resuscitation is of paramount importance during the early stages of sepsis and septic shock management in order to address the deficit in effective vascular volume and the resulting tissue hypoperfusion. Several RCTs have been designed to investigate the timing and amount of fluid administration, as well as to identify appropriate target goals to guide fluid resuscitation (Table 2). Since the ground-breaking EGDT trial by Rivers et al. [50], this has become common practice, and the SSC guidelines currently suggest that at least 30 mL/kg (ideal body weight) of IV crystalloids should be administered within the first 3 h of sepsis-induced hypoperfusion or septic shock [3]. After initial resuscitation, however, fluid administration has to be balanced in order to avoid positive fluid balance and fluid overload, which has been associated with increased mortality, prolonged mechanical ventilation (MV), and worsening of acute kidney injury [98,99,100]. Furthermore, the EGDT approach itself has been under scrutiny, since three RCTs, namely the Australasian Resuscitation in Sepsis Evaluation (ARISE) [101], the Protocolized Care for Early Septic Shock (ProCESS) [102], and the Protocolised Management in Sepsis (ProMISe) [103] trial, showed that the goal of SCVO2 > 70% did not improve mortality, but instead resulted in a worse sequential organ failure assessment (SOFA) score, more days on cardiovascular support, and a longer ICU stay. However, in these trials the pre-randomization administered fluids were close to the 30 mL/kg goal, underscoring the wide adoption of SSC guidelines in the clinical practice [104].
Table 2.
Characteristics of the key randomized controlled trials assessing liberal versus restricted fluid administration in patients with sepsis and septic shock.
| Liberal versus Restricted Fluid Administration in Sepsis | ||||||
|---|---|---|---|---|---|---|
| Study ID | Year | Sample Size | Population | Intervention | Comparison | Outcome |
| EGDT [40] | 2001 | 263 | Adults with sepsis in the ED | Early goal-directed therapy: CVP ≥ 8–12 mmHg, MAP ≥ 65 mmHg, urine ≥ 0.5 mL/kg/h, ScvO2 ≥ 70% N = 130 |
SOC: CVP ≥ 8–12 mmHg, MAP ≥ 65 mmHg, urine ≥ 0.5 mL/kg/h N = 133 |
Significantly lower in-hospital mortality, APACHE II, SAPS II, and MODS in the EGDT group. Patients in EGDT group received more initial fluids, blood transfusions and inotropic support. |
| FEAST [91] | 2011 | 3141 | Children with febrile illness and impaired perfusion | Albumin bolus group N = 1050 Saline bolus group N = 1047 |
No bolus group N = 1044 |
Recruitment was halted due to higher 48 h mortality in the intervention arms, and also, higher 4-week mortality in the bolus groups. |
| ARISE [87] | 2014 | 1588 | Adults with early septic shock in the ED | EGDT N = 796 |
SOC N = 792 |
No difference in 90-day mortality. More patients in the EGDT group received vasopressors, but no other significant differences were observed. |
| ProCESS [88] | 2014 | 902 | Adults in the ED with SIRS and refractory hypotension or hyperlactemia | EGDT N = 456 |
SOC N = 446 |
No difference in 60-day, 90-day, 1-year mortality, or need for organ support |
| ProMISe [89] | 2015 | 1260 | Adults >6 h in the ED with infection, refractory hypotension or hyperlactemia | EGDT N = 630 |
SOC N = 630 |
No difference in 90-daysmortality. Significantly higher cardiovascular support and length of ICU stay in the EGDT group. |
| SSSP-2 [93] | 2017 | 212 | Adults in ED with suspected sepsis and hypotension | Fluid administration guided by SpO2, RR, and JVP (total up to 4 L) N = 107 |
Usual care N = 105 |
Intervention arm received more fluids and vasopressors. Higher in-hospital, 28-day mortality and worsening hypoxemia in the intervention group. |
| FRESH [104] | 2020 | 124 | Adults with sepsis-associated hypotension in ED |
Assessment of fluid responsiveness before fluid administration PLR test, SV change ≥ 10% N = 83 |
Usual care N = 41 |
Similar volume of resuscitation fluids and ICU length of stay in the two arms. Significantly less positive fluid balance, RRT, and MV in the intervention group. |
| CLASSIC [95] | 2022 | 1554 | Adults with septic shock in ICU | Restrictive fluid group Fluids guided by lac, MAP, urine output, mottling, losses, dehydration, and electrolyte disturbances N = 770 |
Liberal fluids according to SOC N = 784 |
No difference in 90-day mortality or serious adverse events between the two group. |
| CLOVERS [96] | 2023 | 1563 | Adults with infection and refractory hypotension | Restrictive fluid group Vasopressor prioritization Only “rescue fluids” for prespecified indications N = 782 |
Liberal fluids according to SOC N = 781 |
No difference in mortality before discharge home by day 90 between the two arms. Similar frequency of adverse events. |
APACHE: Acute Physiology and Chronic Health Evaluation; CVP: central venous pressure; ED: emergency department; EGDT: early goal-directed therapy; ICU: intensive care unit; JVP: jugular venous pressure; MAP: mean arterial pressure; MODS: Multiple Organ Dysfunction Score; MV: mechanical ventilation; PLR: passive leg raising; RR: respiratory rate; RRT: renal replacement therapy; SAPS: Simplified Acute Physiology Score; ScvO2: central venous oxygen saturation; SIRS: systemic inflammatory response syndrome; SOC: standard of care; SpO2: oxygen saturation; SV: stroke volume.
On the other hand, trials conducted in resource-limited settings point towards an excess mortality in patients that received bolus fluid therapy. The Fluid Expansion as Supportive Therapy (FEAST) study, conducted in Africa, found an increased risk of death among children with sepsis who received early treatment with bolus 5% albumin or 0.9% saline, in comparison with the control group [105]. Similarly, the Simplified Severe Sepsis Protocol (SSSP) [106] and the Simplified Severe Sepsis Protocol 2 (SSSP-2) [107] trials, which included African adults with sepsis and hypoperfusion, also showed increased mortality in the intervention arm. The application of an early resuscitation protocol resulted in greater IV fluid administration, vasopressor use, and lactate reduction, but caused worsening hypoxemia and higher mortality, leading to the early termination of the SSSP trial. Of note, the majority of patients in these trials had human immunodeficiency virus (HIV) infection with low CD4+ counts, and were admitted in regular medical wards without access to MV, conditions that prevent the generalization of these results in higher resource settings.
Increased attention has recently been drawn to the optimal fluid management after the initial resuscitation. It has been shown that a higher volume of fluid during the first 3 h, but lower volume in the first 24 h, reduces mortality in severe sepsis and septic shock patients, and that positive total fluid balance increases mortality by 1.7 times [108]. In order to avoid the detrimental effects of fluid overload, further fluid administration should be guided by careful assessment of intravascular volume and organ perfusion [3]. A simple, resource-independent way to assess tissue perfusion is by measuring the capillary refill time (CRT), either on the fingertip or the earlobe [109,110]. In the ANDROMEDA-SHOCK trial, CRT improvement has been found to be a better marker for resuscitation guidance in comparison with the decrease in lactate [111]. In this study, CRT-guided resuscitation resulted in a significantly lower SOFA score at 72 h, and in a lower 28-day mortality that did not reach statistical significance. The ongoing ANDROMEDA-SHOCK-2 trial investigates whether CRT-guided resuscitation based on clinical and hemodynamic phenotypes may decrease mortality in early septic shock patients [112]. The Conservative versus Liberal Approach to Fluid Therapy of Septic Shock in Intensive Care (CLASSIC) [113] and the Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis (CLOVERS) [114] trials were designed to address whether a restrictive versus a liberal approach in fluid management would improve outcomes in patients with sepsis. In the restrictive arm of the studies, additional fluid administration was permitted only if signs of profound hypoperfusion were detected, in terms of lactate levels > 4 mmol/L, MAP < 50 mmHg, extensive mottling, or urine output < 0.1 mL/kg/h. In both trials, there was no difference detected in the primary outcome of 90-day mortality. Furthermore, a meta-analysis of studies evaluating a restrictive versus a liberal fluid strategy after initial resuscitation in septic patients found that the former was associated with a lower duration of MV, but had no effect on mortality [115]. A previous meta-analysis provided similar results also including acute respiratory distress syndrome (ARDS) and systemic inflammatory response syndrome (SIRS) in addition to sepsis management [116]. Nevertheless, the question remains whether there are specific subgroups of patients who would benefit from a more aggressive or a more conservative IV fluid administration, and if there is a reliable approach to identify them.
Evaluating the heart’s flow response to fluid administration has been proposed to differentiate between fluid responsive and fluid refractory septic states. As static measures, such as CVP, are poor indices of fluid status [24,117], dynamic measures have been employed to predict fluid responsiveness [3,118,119]. Dynamic metrics include passive leg raising (PLR) along with cardiac output (CO) assessment, pulse contour analysis, pulse pressure variation (PPV), stroke volume variation (SVV), and inferior vena cava (IVC) variability with respiration [120]. Passive leg raising to 45° produces hemodynamic changes that mimic volume expansion, and in preload-dependent states, it produces an increase in CO. A >10% increase in CO reliably predicts a fluid-responsive state [121]. In the Fluid Responsiveness Evaluation in Sepsis-associated Hypotension (FRESH) study [122], the researchers compared the use of PLR to guide fluid administration against usual care, and found that the intervention group showed a lower net fluid balance, as well as reduced requirement for renal replacement therapy and MV. The increase in stroke volume (SV) was assessed by a non-invasive bioreactance technology, which has been validated against the more invasive thermodilution method [123,124]. A meta-analysis has confirmed that the PLR challenge detects fluid responsiveness with high sensitivity and specificity [121].
In intubated patients, changes in intrathoracic pressure during MV can be used for the dynamic assessment of fluid responsiveness. Pulse pressure and stroke volume changes between inspiration and expiration, assessed by means of pressure/pulse waveform analysis, have been associated with intravascular volume status and the probability that a volume challenge will increase the SV [125,126]. However, the reliability of these metrics can be limited in certain situations, commonly in the ICU setting, such as the presence of spontaneous breathing, cardiac arrythmias, tidal volume < 8 mL/kg, and reduced lung compliance [127,128]. Doppler echocardiography can also be employed to detect SV changes through aortic velocity time integral measurement, upon bolus fluid administration or PLR manoeuvre, and usually is the most readily available method [129]. Although IVC diameter variation can be easily assessed and has been used to determine preload dependence, reports show that it has limited sensitivity and specificity [130]. Moreover, the standard subcostal measurement of IVC diameter is not always feasible, due to obesity, bowel distention, or presence of surgical wounds. The alternative trans-hepatic approach has been used in these cases, but the results obtained by these two methods are not interchangeable [131]. The measurement of the superior vena cava respiratory variation, although more accurate, it requires the use of transoesophageal Doppler by experienced personnel [132].
Since findings suggest that fluid restriction and negative fluid balance benefits patients with ARDS, whereas liberal fluids are associated with prolonged MV, extravascular lung water (EVLW) measurement has been suggested to guide fluid administration in addition to dynamic measures [128]. EVLW, a marker of pulmonary edema, vascular permeability, and ARDS, is ideally measured by transpulmonary thermodilution, but when not available, lung ultrasonography may provide a rough estimate of lung congestion. In the presence of fluid responsiveness, a fluid challenge will lead to a small increase in EVLW, but in non-responsive states, further fluid administration will result in a large increase in EVLW [13]. A high EVLW during resuscitation informs the physician to maximize efforts towards fluid restriction and to shift to an alternative method for hemodynamic stabilization [133].
Dynamic assessment of fluid responsiveness in a meta-analysis has been associated with a decrease in mortality, ICU length of stay, and duration of MV, but only one study with septic patients was included [134]. In this study, no difference was found in time-to-shock resolution between preload dependence (PLR/PPV) and the control (CVP) arm. A more recent meta-analysis failed to prove a mortality benefit from fluid responsiveness guided treatment in septic patients [52,135]. Consequently, although dynamic assessment has been shown to predict volume responsiveness in sepsis, this has yet to be associated with increased survival, and more studies are needed to assess whether this strategy improves patient-important outcomes.
6. Conclusions and Future Directions
Until recently, efforts have been mainly focused on the assessment of macrocircula-tion, such as cardiac output and blood pressure, to guide fluid resuscitation in patients with sepsis, with the exception of CRT. However, the assessment of microcirculation parameters, in relation to microvascular flow and density, tissue perfusion and oxygenation, and glycocalyx integrity has been on the epicenter of intensive research with promising results. Hand-held vital microscopes (HVM) have been investigated for the visualization of the sublingual capillary network, providing a wealth of information in relation to blood flow characteristics, vascular density, and glycocalyx [136,137]. Contrast-enhanced ultrasound (CEUS) is a promising technique for the assessment of the effects of fluid resuscitation and vasopressor administration on the renal microcirculation, as it holds the benefit of directly assessing organ perfusion [138,139]. New methods have also been under research for the assessment of peripheral perfusion, such as laser Doppler flowmetry, a skin blood flow measurement tool [140]. It has been found to correlate well with ICU mortality, and is yet to be tested as a tool to guide resuscitation efforts [141]. These and other methods have been recently reviewed elsewhere [142].
Although fluid management in the septic patient has been extensively studied during the last decades, the “which”, “when”, and “how much” questions are still to be addressed. Evidently, fluid resuscitation has been associated with both benefits and harm; however, there is still a paucity of high-quality evidence to guide the clinical practice regarding fluid management in sepsis. Therefore, controversies remain in the scientific community, and more high-quality, robust studies are warranted to guide future developments. The “one size fits all” approach may be convenient, but it seems that personalized fluid management and taking patient-specific hemodynamic indices into account will provide the basis for better patient outcomes in the future. In this respect, cutting-edge tools for the assessment of perfusion on the tissue level are being investigated and their implementation in real-world settings, however challenging, is expected to revolutionize the management of these patients. Well-designed RCTs are required to compare fluid choices, specifically in septic patients, and they have to be powered enough to evaluate patient subgroups that may respond differently to different types or volume of fluids administered. Existing diagnostic tools have been proven to be valuable in assessing fluid responsiveness, although further trials are yet to determine improvement in patient-important outcomes.
The take-home messages are as follows:
Data on the optimal type (balanced crystalloids versus normal saline) and volume (liberal versus restricted administration) of fluids in sepsis and septic shock patients are still controversial and elusive.
Current SSC guidelines recommend the early administration of 30 mL/kg of IV crystalloid fluids for sepsis-related hypotension or a lactate ≥ 4 mmol/L, within the first 3 h of resuscitation. This is a weak recommendation and is based on low-quality evidence.
Regarding the type of fluid administered during resuscitation, the majority of clinical trials demonstrated no significant difference between the balanced crystalloids and normal saline in the acute kidney injury and mortality.
Excessive fluid administration during resuscitation can lead to worse outcomes in the septic patient.
Fluid administration after initial resuscitation should be preferably guided by dynamic measures of fluid responsiveness.
Fluid management in the critical patient can be optimized by the personalized, bedside, and dynamic assessment of macro- and microcirculation indices.
Abbreviations
| AKI | Acute kidney injury |
| ALBIOS | Albumin Italian Outcome Sepsis |
| APACHE | Acute Physiology and Chronic Health Evaluation |
| ARDS | Acute respiratory distress syndrome |
| BaSICS | Balanced Solution in Intensive Care Study |
| BS | Balanced Solution |
| CEUS | Contrast-enhanced ultrasound |
| CLASSIC | Conservative versus Liberal Approach to Fluid Therapy of Septic Shock in Intensive Care |
| CLOVERS | Crystalloid Liberal or Vasopressors Early Resuscitation in Sepsis |
| CO | Cardiac output |
| CRT | Capillary refill time |
| CVP | Central venous pressure |
| DAMPs | Danger-associated molecular patterns |
| ED | Emergency department |
| EGDT | Early goal-directed therapy |
| ESICM | European Society of Intensive Care Medicine |
| EVLW | Extravascular lung water |
| FEAST | Fluid Expansion as Supportive Therapy |
| FRESH | Fluid Responsiveness Evaluation in Sepsis-associated Hypotension |
| ICU | Intensive care unit |
| HES | Hydroxyethyl starch |
| HIV | Human immunodeficiency virus |
| HVM | Hand-held vital microscopes |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| IV | Intravenous |
| IVC | Inferior vena cava |
| JVP | Jugular venous pressure |
| MAP | Mean arterial pressure |
| MODS | Multiple Organ Dysfunction Score |
| MV | Mechanical ventilation |
| NS | Normal saline |
| PAMPs | Pathogen-associated molecular patterns |
| PLR | Passive leg raising |
| PLUS | Plasma-Lyte 148 versus Saline Study |
| PPV | Pulse pressure variation |
| ProCESS | Protocolized Care for Early Septic Shock |
| ProMISe | Protocolised Management in Sepsis |
| PRoMPT BOLUS | Pragmatic Pediatric trial of Balanced versus NS Fluid in sepsis |
| PRRs | Pattern recognition receptors |
| RCTs | Randomized controlled trials |
| ROSE | Resuscitation, optimization, stabilization, and evacuation |
| RR | Respiratory rate |
| RRT | Renal replacement therapy |
| SAFE | Saline versus Albumin Fluid Evaluation |
| SALT | Isotonic Solution Administration Logistical Testing |
| SAPS | Simplified Acute Physiology Score |
| ScvO2 | Central venous oxygen saturation |
| SIRS | Systemic inflammatory response syndrome |
| SMART | Isotonic Solutions and Major Adverse Renal Events Trial |
| SOC | Standard of care |
| SOFA | Sequential organ failure assessment |
| SPLIT | Saline vs. Plasma-Lyte 148 for ICU fluid Therapy |
| SpO2 | Oxygen saturation |
| SSC | Surviving Sepsis Campaign |
| SSSP | Simplified Severe Sepsis Protocol |
| SSSP-2 | Simplified Severe Sepsis Protocol 2 |
| SV | Stroke volume |
| SVR | Systemic vascular resistance |
| SVV | Stroke volume variation |
| TLRs | Toll-like receptors |
| TNF-a | Tumor necrosis factor alpha |
Author Contributions
Conceptualization: D.D., P.C.F. and C.D.M.; investigation: D.D., C.D.M., A.D. and K.D.; writing-draft preparation: D.D., C.D.M., A.D. and K.D.; writing-review and editing: D.D., C.D.M., P.C.F., K.P., S.T., A.K. and N.Z.; administration: D.D. and C.D.M.; supervision: P.C.F., K.P., S.T., A.K. and N.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G.R., Chiche J.-D., Coopersmith C.M., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans L., Rhodes A., Alhazzani W., Antonelli M., Coopersmith C.M., French C., Machado F.R., Mcintyre L., Ostermann M., Prescott H.C., et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensiv. Care Med. 2021;47:1181–1247. doi: 10.1007/s00134-021-06506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hotchkiss R.S., Karl I.E. The Pathophysiology and Treatment of Sepsis. N. Engl. J. Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 5.Angus D.C., van der Poll T. Severe Sepsis and Septic Shock. N. Engl. J. Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 7.De Backer D., Cecconi M., Lipman J., Machado F., Myatra S.N., Ostermann M., Perner A., Teboul J.-L., Vincent J.-L., Walley K.R. Challenges in the management of septic shock: A narrative review. Intensiv. Care Med. 2019;45:420–433. doi: 10.1007/s00134-019-05544-x. [DOI] [PubMed] [Google Scholar]
- 8.Gavelli F., Castello L.M., Avanzi G.C. Management of sepsis and septic shock in the emergency department. Intern. Emerg. Med. 2021;16:1649–1661. doi: 10.1007/s11739-021-02735-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown R.M., Semler M.W. Fluid Management in Sepsis. J. Intensiv. Care Med. 2019;34:364–373. doi: 10.1177/0885066618784861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macdonald S. Fluid Resuscitation in Patients Presenting with Sepsis: Current Insights. Open Access Emerg. Med. 2022;14:633–638. doi: 10.2147/OAEM.S363520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ladzinski A.T., Thind G.S., Siuba M.T. Rational Fluid Resuscitation in Sepsis for the Hospitalist: A Narrative Review. Mayo Clin. Proc. 2021;96:2464–2473. doi: 10.1016/j.mayocp.2021.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Monnet X., Teboul J.-L. My patient has received fluid. How to assess its efficacy and side effects? Ann. Intensiv. Care. 2018;8:54. doi: 10.1186/s13613-018-0400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marik P., Bellomo R. A rational approach to fluid therapy in sepsis. Br. J. Anaesth. 2016;116:339–349. doi: 10.1093/bja/aev349. [DOI] [PubMed] [Google Scholar]
- 14.Bissell B.D., Mefford B. Pathophysiology of Volume Administration in Septic Shock and the Role of the Clinical Pharmacist. Ann. Pharmacother. 2020;54:388–396. doi: 10.1177/1060028019887160. [DOI] [PubMed] [Google Scholar]
- 15.Ueyama H., Kiyonaka S. Predicting the Need for Fluid Therapy—Does Fluid Responsiveness Work? J. Intensiv. Care. 2017;5:34. doi: 10.1186/s40560-017-0210-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marik P.E., Lemson J. Fluid responsiveness: An evolution of our understanding. Br. J. Anaesth. 2014;112:617–620. doi: 10.1093/bja/aet590. [DOI] [PubMed] [Google Scholar]
- 17.Malbrain M.L.N.G., Van Regenmortel N., Saugel B., De Tavernier B., Van Gaal P.-J., Joannes-Boyau O., Teboul J.-L., Rice T.W., Mythen M., Monnet X. Principles of fluid management and stewardship in septic shock: It is time to consider the four D’s and the four phases of fluid therapy. Ann. Intensiv. Care. 2018;8:66. doi: 10.1186/s13613-018-0402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lundy D.J., Trzeciak S. Microcirculatory Dysfunction in Sepsis. Crit. Care Clin. 2009;25:721–731. doi: 10.1016/j.ccc.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Marik P.E., Byrne L., van Haren F. Fluid resuscitation in sepsis: The great 30 mL per kg hoax. J. Thorac. Dis. 2020;12:S37–S47. doi: 10.21037/jtd.2019.12.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrne L., Van Haren F. Fluid resuscitation in human sepsis: Time to rewrite history? Ann. Intensiv. Care. 2017;7:4. doi: 10.1186/s13613-016-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byrne L., Byrne L., Obonyo N.G., Obonyo N.G., Diab S.D., Diab S.D., Dunster K.R., Dunster K.R., Passmore M.R., Passmore M.R., et al. Unintended Consequences: Fluid Resuscitation Worsens Shock in an Ovine Model of Endotoxemia. Am. J. Respir. Crit. Care Med. 2018;198:1043–1054. doi: 10.1164/rccm.201801-0064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maeder M., Fehr T., Rickli H., Ammann P. Sepsis-associated myocardial dysfunction: Diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest. 2006;129:1349–1366. doi: 10.1378/chest.129.5.1349. [DOI] [PubMed] [Google Scholar]
- 23.Bessière F., Khenifer S., Dubourg J., Durieu I., Lega J.-C. Prognostic value of troponins in sepsis: A meta-analysis. Intensiv. Care Med. 2013;39:1181–1189. doi: 10.1007/s00134-013-2902-3. [DOI] [PubMed] [Google Scholar]
- 24.Marik P.E., Cavallazzi R. Does the Central Venous Pressure Predict Fluid Responsiveness? An Updated Meta-Analysis and a Plea for Some Common Sense. Crit. Care Med. 2013;41:1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- 25.Leisman D., Doerfler M.E., Schneider S.M., Masick K.D., D’amore J.A., D’angelo J.K. Predictors, Prevalence, and Outcomes of Early Crystalloid Responsiveness Among Initially Hypotensive Patients With Sepsis and Septic Shock. Crit. Care Med. 2018;46:189–198. doi: 10.1097/CCM.0000000000002834. [DOI] [PubMed] [Google Scholar]
- 26.Rhodes A., Lamb F.J., Malagon I., Newman P.J., Grounds R.M., Bennett E.D. A prospective study of the use of a dobutamine stress test to identify outcome in patients with sepsis, severe sepsis, or septic shock. Crit. Care Med. 1999;27:2361–2366. doi: 10.1097/00003246-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Losser M.-R., Forget A.-P., Payen D. Nitric oxide involvement in the hemodynamic response to fluid resuscitation in endotoxic shock in rats. Crit. Care Med. 2006;34:2426–2431. doi: 10.1097/01.CCM.0000231878.82244.C9. [DOI] [PubMed] [Google Scholar]
- 28.García M.I.M., González P.G., Romero M.G., Gil Cano A., Oscier C., Rhodes A., Grounds R.M., Cecconi M. Effects of fluid administration on arterial load in septic shock patients. Intensiv. Care Med. 2015;41:1247–1255. doi: 10.1007/s00134-015-3898-7. [DOI] [PubMed] [Google Scholar]
- 29.Pohl U., De Wit C., Gloe T. Large arterioles in the control of blood flow: Role of endothelium-dependent dilation. Acta Physiol. Scand. 2000;168:505–510. doi: 10.1046/j.1365-201x.2000.00702.x. [DOI] [PubMed] [Google Scholar]
- 30.Malbrain M.L., Marik P.E., Witters I., Cordemans C., Kirkpatrick A.W., Roberts D.J., Van Regenmortel N. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: A systematic review with suggestions for clinical practice. Anaesthesiol. Intensiv. Ther. 2014;46:361–380. doi: 10.5603/AIT.2014.0060. [DOI] [PubMed] [Google Scholar]
- 31.Samoni S., Vigo V., Reséndiz L.I.B., Villa G., De Rosa S., Nalesso F., Ferrari F., Meola M., Brendolan A., Malacarne P., et al. Impact of hyperhydration on the mortality risk in critically ill patients admitted in intensive care units: Comparison between bioelectrical impedance vector analysis and cumulative fluid balance recording. Crit. Care. 2016;20:95. doi: 10.1186/s13054-016-1269-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Landry D.W., Oliver J.A. The Pathogenesis of Vasodilatory Shock. N. Engl. J. Med. 2001;345:588–595. doi: 10.1056/NEJMra002709. [DOI] [PubMed] [Google Scholar]
- 33.Sadaka F., Juarez M., Naydenov S., O’brien J. Fluid resuscitation in septic shock: The effect of increasing fluid balance on mortality. J. Intensiv. Care Med. 2013;29:213–217. doi: 10.1177/0885066613478899. [DOI] [PubMed] [Google Scholar]
- 34.Edwards M.R., Mythen M.G. Fluid therapy in critical illness. Extrem. Physiol. Med. 2014;3:16. doi: 10.1186/2046-7648-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uchimido R., Schmidt E.P., Shapiro N.I. The glycocalyx: A novel diagnostic and therapeutic target in sepsis. Crit. Care. 2019;23:16. doi: 10.1186/s13054-018-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chappell D., Bruegger D., Potzel J., Jacob M., Brettner F., Vogeser M., Conzen P., Becker B.F., Rehm M. Hypervolemia increases release of atrial natriuretic peptide and shedding of the endothelial glycocalyx. Crit. Care. 2014;18:538. doi: 10.1186/s13054-014-0538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacob M., Saller T., Chappell D., Rehm M., Welsch U., Becker B.F. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res. Cardiol. 2013;108:347. doi: 10.1007/s00395-013-0347-z. [DOI] [PubMed] [Google Scholar]
- 38.Bihari S., Prakash S., Bersten A.D. Post resusicitation fluid boluses in severe sepsis or septic shock: Prevalence and efficacy (price study) Shock. 2013;40:28–34. doi: 10.1097/SHK.0b013e31829727f1. [DOI] [PubMed] [Google Scholar]
- 39.Glassford N.J., Eastwood G.M., Bellomo R. Physiological changes after fluid bolus therapy in sepsis: A systematic review of contemporary data. Crit. Care. 2014;18:696. doi: 10.1186/s13054-014-0696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodcock T.E., Woodcock T.M. Revised Starling equation and the glycocalyx model of transvascular fluid exchange: An improved paradigm for prescribing intravenous fluid therapy. Br. J. Anaesth. 2012;108:384–394. doi: 10.1093/bja/aer515. [DOI] [PubMed] [Google Scholar]
- 41.Van Regenmortel N., Verbrugghe W., Roelant E., Wyngaert T.V.D., Jorens P.G. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: A retrospective study in a tertiary mixed ICU population. Intensiv. Care Med. 2018;44:409–417. doi: 10.1007/s00134-018-5147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Regenmortel N., Hendrickx S., Roelant E., Baar I., Dams K., Van Vlimmeren K., Embrecht B., Wittock A., Hendriks J.M., Lauwers P., et al. 154 compared to 54 mmol per liter of sodium in intravenous maintenance fluid therapy for adult patients undergoing major thoracic surgery (TOPMAST): A single-center randomized controlled double-blind trial. Intensiv. Care Med. 2019;45:1422–1432. doi: 10.1007/s00134-019-05772-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dalfino L., Tullo L., Donadio I., Malcangi V., Brienza N. Intra-abdominal hypertensionand acute renal failurein critically ill patients. Intensiv. Care Med. 2008;34:707–713. doi: 10.1007/s00134-007-0969-4. [DOI] [PubMed] [Google Scholar]
- 44.Diebel L.N., Wilson R.F., Dulchavsky S.A., Saxe J. Effect of Increased Intra-Abdominal Pressure on Hepatic Arterial, Portal Venous, and Hepatic Microcirculatory Blood Flow. J. Trauma. 1992;33:273–279. doi: 10.1097/00005373-199208000-00019. [DOI] [PubMed] [Google Scholar]
- 45.Scheeren T.W.L., Bakker J., De Backer D., Annane D., Asfar P., Boerma E.C., Cecconi M., Dubin A., Dünser M.W., Duranteau J., et al. Current use of vasopressors in septic shock. Ann. Intensiv. Care. 2019;9:20. doi: 10.1186/s13613-019-0498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levy M.M., Evans L.E., Rhodes A. The Surviving Sepsis Campaign Bundle: 2018 update. Intensiv. Care Med. 2018;44:925–928. doi: 10.1007/s00134-018-5085-0. [DOI] [PubMed] [Google Scholar]
- 47.Mukherjee V., Evans L. Implementation of the Surviving Sepsis Campaign guidelines. Curr. Opin. Crit. Care. 2017;23:412–416. doi: 10.1097/MCC.0000000000000438. [DOI] [PubMed] [Google Scholar]
- 48.Cosnett J. The Origins of Intravenous Fluid Therapy. Lancet. 1989;333:768–771. doi: 10.1016/S0140-6736(89)92583-X. [DOI] [PubMed] [Google Scholar]
- 49.Daniels R. Surviving the first hours in sepsis: Getting the basics right (an intensivist’s perspective) J. Antimicrob. Chemother. 2011;66((Suppl. 2)):ii11–ii23. doi: 10.1093/jac/dkq515. [DOI] [PubMed] [Google Scholar]
- 50.Rivers E., Nguyen B., Havstad S., Ressler J., Muzzin A., Knoblich B., Peterson E., Tomlanovich M. Early Goal-Directed Therapy in the Treatment of Severe Sepsis and Septic Shock. N. Engl. J. Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 51.Angus D.C., Barnato A.E., Bell D., Bellomo R., Chong C.-R., Coats T.J., Davies A., Delaney A., Harrison D.A., Holdgate A., et al. A systematic review and meta-analysis of early goal-directed therapy for septic shock: The ARISE, ProCESS and ProMISe Investigators. Intensiv. Care Med. 2015;41:1549–1560. doi: 10.1007/s00134-015-3822-1. [DOI] [PubMed] [Google Scholar]
- 52.Ehrman R.R., Gallien J.Z., Smith R.K., Akers K., Malik A.N., Harrison N., Welch R.D., Levy P.D., Sherwin R.L. Resuscitation Guided by Volume Responsiveness Does Not Reduce Mortality in Sepsis: A Meta-Analysis. Crit. Care Explor. 2019;1:e0015. doi: 10.1097/CCE.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith S.H., Perner A. Higher vs. lower fluid volume for septic shock: Clinical characteristics and outcome in unselected patients in a prospective, multicenter cohort. Crit. Care. 2012;16:R76. doi: 10.1186/cc11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McIntyre L., Rowe B.H., Walsh T.S., Gray A., Arabi Y., Perner A., Gordon A., Marshall J., Cook D., Fox-Robichaud A., et al. Multicountry survey of emergency and critical care medicine physicians’ fluid resuscitation practices for adult patients with early septic shock. BMJ Open. 2016;6:e010041. doi: 10.1136/bmjopen-2015-010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoste E.A., Maitland K., Brudney C.S., Mehta R., Vincent J.-L., Yates D., Kellum J.A., Mythen M.G., Shaw A.D. Four phases of intravenous fluid therapy: A conceptual model. Br. J. Anaesth. 2014;113:740–747. doi: 10.1093/bja/aeu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Malbrain M.L.N.G., Van Regenmortel N., Owczuk R. It is time to consider the four D’s of fluid management. Anaesthesiol. Intensiv. Ther. 2015;47:s1–s5. doi: 10.5603/AIT.a2015.0070. [DOI] [PubMed] [Google Scholar]
- 57.Brunkhorst F.M., Engel C., Bloos F., Meier-Hellmann A., Ragaller M., Weiler N., Moerer O., Gruendling M., Oppert M., Grond S., et al. Intensive Insulin Therapy and Pentastarch Resuscitation in Severe Sepsis. N. Engl. J. Med. 2008;358:125–139. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 58.Guidet B., Martinet O., Boulain T., Philippart F., Poussel J., Maizel J., Forceville X., Feissel M., Hasselmann M., Heininger A., et al. Assessment of hemodynamic efficacy and safety of 6% hydroxyethylstarch 130/0.4 vs. 0.9% NaCl fluid replacement in patients with severe sepsis: The CRYSTMAS study. Crit. Care. 2012;16:R94. doi: 10.1186/11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perner A., Haase N., Guttormsen A.B., Tenhunen J., Klemenzson G., Åneman A., Madsen K.R., Møller M.H., Elkjær J.M., Poulsen L.M., et al. Hydroxyethyl Starch 130/0.42 versus Ringer’s Acetate in Severe Sepsis. N. Engl. J. Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 60.Myburgh J.A., Finfer S., Bellomo R., Billot L., Cass A., Gattas D., Glass P., Lipman J., Liu B., McArthur C., et al. Hydroxyethyl Starch or Saline for Fluid Resuscitation in Intensive Care. N. Engl. J. Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 61.Zarychanski R., Abou-Setta A.M., Turgeon A.F., Houston B., McIntyre L., Marshall J.C., Fergusson D. Association of Hydroxyethyl Starch Administration With Mortality and Acute Kidney Injury in Critically Ill Patients Requiring Volume Resuscitation: A systematic review and meta-analysis. JAMA. 2013;309:678–688. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 62.Moeller C., Fleischmann C., Thomas-Rueddel D., Vlasakov V., Rochwerg B., Theurer P., Gattinoni L., Reinhart K., Hartog C.S. How safe is gelatin? A systematic review and meta-analysis of gelatin-containing plasma expanders vs crystalloids and albumin. J. Crit. Care. 2016;35:75–83. doi: 10.1016/j.jcrc.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 63.Finfer S., McEvoy S., Bellomo R., McArthur C., Myburgh J., Norton R. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensiv. Care Med. 2011;37:86–96. doi: 10.1007/s00134-010-2039-6. [DOI] [PubMed] [Google Scholar]
- 64.Caironi P., Tognoni G., Masson S., Fumagalli R., Pesenti A., Romero M., Fanizza C., Caspani L., Faenza S., Grasselli G., et al. Albumin Replacement in Patients with Severe Sepsis or Septic Shock. N. Engl. J. Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 65.Xu J.-Y., Chen Q.-H., Xie J.-F., Pan C., Liu S.-Q., Huang L.-W., Yang C.-S., Liu L., Huang Y.-Z., Guo F.-M., et al. Comparison of the effects of albumin and crystalloid on mortality in adult patients with severe sepsis and septic shock: A meta-analysis of randomized clinical trials. Crit. Care. 2014;18:702. doi: 10.1186/s13054-014-0702-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chien S.-C., Chen C.-Y., Lin C.-F., Yeh H.-I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark. Res. 2017;5:31. doi: 10.1186/s40364-017-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Casey J.D., Brown R.M., Semler M.W. Resuscitation fluids. Curr. Opin. Crit. Care. 2018;24:512–518. doi: 10.1097/MCC.0000000000000551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Winters M.E., Sherwin R., Vilke G.M., Wardi G. What is the Preferred Resuscitation Fluid for Patients with Severe Sepsis and Septic Shock? J. Emerg. Med. 2017;53:928–939. doi: 10.1016/j.jemermed.2017.08.093. [DOI] [PubMed] [Google Scholar]
- 69.Seifter J.L. Integration of Acid–Base and Electrolyte Disorders. N. Engl. J. Med. 2015;372:389–392. doi: 10.1056/NEJMra1215672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gruartmoner G., Mesquida J., Ince C. Fluid therapy and the hypovolemic microcirculation. Curr. Opin. Crit. Care. 2015;21:276–284. doi: 10.1097/MCC.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 71.Kozek-Langenecker S.A. Fluids and coagulation. Curr. Opin. Crit. Care. 2015;21:285–291. doi: 10.1097/MCC.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 72.Funke B.E., Jackson K.E., Self W.H., Collins S.P., Saunders C.T., Wang L., Blume J.D., Wickersham N., Brown R.M., Casey J.D., et al. Effect of balanced crystalloids versus saline on urinary biomarkers of acute kidney injury in critically ill adults. BMC Nephrol. 2021;22:54. doi: 10.1186/s12882-021-02236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou F., Peng Z.-Y., Bishop J.V.B., Cove M.E., Singbartl K., Kellum J.A.M. Effects of Fluid Resuscitation With 0.9% Saline Versus a Balanced Electrolyte Solution on Acute Kidney Injury in a Rat Model of Sepsis. Crit. Care Med. 2014;42:e270–e278. doi: 10.1097/CCM.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jaynes M.P., Murphy C.V., Ali N., Krautwater A., Lehman A., Doepker B.A. Association between chloride content of intravenous fluids and acute kidney injury in critically ill medical patients with sepsis. J. Crit. Care. 2018;44:363–367. doi: 10.1016/j.jcrc.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 75.Myburgh J.A., Mythen M.G. Resuscitation fluids. N. Engl. J. Med. 2013;369:1243–1251. doi: 10.1056/NEJMra1208627. [DOI] [PubMed] [Google Scholar]
- 76.Morgan T.J. The ideal crystalloid what is ‘balanced’? Curr. Opin. Crit. Care. 2013;19:299–307. doi: 10.1097/MCC.0b013e3283632d46. [DOI] [PubMed] [Google Scholar]
- 77.Singh S., Kerndt C.C., Davis D. Statpearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Ringer’s Lactate. [PubMed] [Google Scholar]
- 78.Rizoli S. PlasmaLyte. J. Trauma. 2011;70:S17–S18. doi: 10.1097/TA.0b013e31821a4d89. [DOI] [PubMed] [Google Scholar]
- 79.Duffy R.A., Foroozesh M.B., Loflin R.D., Ie S.R., Icard B.L., Tegge A.N., Nogueira J.R., Kuehl D.R., Smith D.C., Loschner A.L. Normal saline versus Normosol™-R in sepsis resuscitation: A retrospective cohort study. J. Intensiv. Care Soc. 2019;20:223–230. doi: 10.1177/1751143718786113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Young P., Bailey M., Beasley R., Henderson S., Mackle D., McArthur C., McGuinness S., Mehrtens J., Myburgh J., Psirides A., et al. Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA. 2015;314:1701–1710. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 81.Semler M.W., Wanderer J.P., Ehrenfeld J.M., Stollings J.L., Self W.H., Siew E.D., Wang L., Byrne D.W., Shaw A.D., Bernard G.R., et al. Balanced Crystalloids versus Saline in the Intensive Care Unit. The SALT Randomized Trial. Am. J. Respir. Crit. Care Med. 2017;195:1362–1372. doi: 10.1164/rccm.201607-1345OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Semler M.W., Self W.H., Wanderer J.P., Ehrenfeld J.M., Wang L., Byrne D.W., Stollings J.L., Kumar A.B., Hughes C.G., Hernandez A., et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brown R.M., Wang L., Coston T.D., Krishnan N.I., Casey J.D., Wanderer J.P., Ehrenfeld J.M., Byrne D.W., Stollings J.L., Siew E.D., et al. Balanced Crystalloids versus Saline in Sepsis. A Secondary Analysis of the SMART Clinical Trial. Am. J. Respir. Crit. Care Med. 2019;200:1487–1495. doi: 10.1164/rccm.201903-0557OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zampieri F.G., Machado F.R., Biondi R.S., Freitas F.G.R., Veiga V.C., Figueiredo R.C., Lovato W.J., Amêndola C.P., Serpa-Neto A., Paranhos J.L.R., et al. Effect of Intravenous Fluid Treatment With a Balanced Solution vs 0.9% Saline Solution on Mortality in Critically Ill Patients: The BaSICS Randomized Clinical Trial. JAMA. 2021;326:818–829. doi: 10.1001/jama.2021.11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zampieri F.G., Machado F.R., Biondi R.S., Freitas F.G.R., Veiga V.C., Figueiredo R.C., Lovato W.J., Amêndola C.P., Serpa-Neto A., Paranhos J.L.R., et al. Association between Type of Fluid Received Prior to Enrollment, Type of Admission, and Effect of Balanced Crystalloid in Critically Ill Adults: A Secondary Exploratory Analysis of the BaSICS Clinical Trial. Am. J. Respir. Crit. Care Med. 2022;205:1419–1428. doi: 10.1164/rccm.202111-2484OC. [DOI] [PubMed] [Google Scholar]
- 86.Finfer S., Micallef S., Hammond N., Navarra L., Bellomo R., Billot L., Delaney A., Gallagher M., Gattas D., Li Q., et al. Balanced Multielectrolyte Solution versus Saline in Critically Ill Adults. N. Engl. J. Med. 2022;386:815–826. doi: 10.1056/NEJMoa2114464. [DOI] [PubMed] [Google Scholar]
- 87.Limapichat T., Pattanapong K. Normal Saline Solution or Lactated Ringer’s Solution to Enhance Lactate Clearance in Septic Patients After Initial Resuscitation in the ED: A Retrospective Cohort Trial. Open Access Emerg. Med. 2021;13:511–519. doi: 10.2147/OAEM.S340691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Raghunathan K., Shaw A., Nathanson B., Stürmer T., Brookhart A., Stefan M., Setoguchi S., Beadles C., Lindenauer P.K. Association Between the Choice of IV Crystalloid and In-Hospital Mortality Among Critically Ill Adults With Sepsis. Crit. Care Med. 2014;42:1585–1591. doi: 10.1097/CCM.0000000000000305. [DOI] [PubMed] [Google Scholar]
- 89.Corrêa T.D., Cavalcanti A.B., De Assunção M.S.C. Balanced crystalloids for septic shock resuscitation. Rev. Bras. Ter. Intensiv. 2016;28:463–471. doi: 10.5935/0103-507X.20160079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schindler A.W., Marx G. Evidence-based fluid management in the ICU. Curr. Opin. Anaesthesiol. 2016;29:158–165. doi: 10.1097/ACO.0000000000000303. [DOI] [PubMed] [Google Scholar]
- 91.Rochwerg B., Alhazzani W., Gibson A., Ribic C.M., Sindi A., Heels-Ansdell D., Thabane L., Fox-Robichaud A., Mbuagbaw L., Szczeklik W., et al. Fluid type and the use of renal replacement therapy in sepsis: A systematic review and network meta-analysis. Intensiv. Care Med. 2015;41:1561–1571. doi: 10.1007/s00134-015-3794-1. [DOI] [PubMed] [Google Scholar]
- 92.Xue M., Zhang X., Liu F., Chang W., Xie J., Xu J., Yang Y., Qiu H. Effects of chloride content of intravenous crystalloid solutions in critically ill adult patients: A meta-analysis with trial sequential analysis of randomized trials. Ann. Intensiv. Care. 2019;9:30. doi: 10.1186/s13613-019-0506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu Y., Guo N.M., Song M.M., Xia F.M., Wu Y., Wang X.M., Chen T.M., Yang Z.M., Yang S.M., Zhang Y.M., et al. Balanced crystalloids versus saline in critically ill patients: The PRISMA study of a meta-analysis. Medicine. 2021;100:e27203. doi: 10.1097/MD.0000000000027203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hammond D.A., Lam S.W., Rech M.A., Smith M.N., Westrick J., Trivedi A.P., Balk R.A. Balanced Crystalloids Versus Saline in Critically Ill Adults: A Systematic Review and Meta-analysis. Ann. Pharmacother. 2020;54:5–13. doi: 10.1177/1060028019866420. [DOI] [PubMed] [Google Scholar]
- 95.Beran A., Altorok N., Srour O., Malhas S.-E., Khokher W., Mhanna M., Ayesh H., Aladamat N., Abuhelwa Z., Srour K., et al. Balanced Crystalloids versus Normal Saline in Adults with Sepsis: A Comprehensive Systematic Review and Meta-Analysis. J. Clin. Med. 2022;11:1971. doi: 10.3390/jcm11071971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Castro R.E.V., Medeiros D.N.M., Prata-Barbosa A., de Magalhães-Barbosa M.C. Surviving Sepsis Campaign International Guidelines for the Management of Septic Shock and Sepsis-Associated Organ Dysfunction in Children. Pediatr. Crit. Care Med. 2020;21:924–925. doi: 10.1097/PCC.0000000000002444. [DOI] [PubMed] [Google Scholar]
- 97.Weiss S.L., Balamuth F., Long E., Thompson G.C., Hayes K.L., Katcoff H., Cook M., Tsemberis E., Hickey C.P., Williams A., et al. PRagMatic Pediatric Trial of Balanced vs nOrmaL Saline FlUid in Sepsis: Study protocol for the PRoMPT BOLUS randomized interventional trial. Trials. 2021;22:776. doi: 10.1186/s13063-021-05717-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boyd J.H., Forbes J., Nakada T.-A., Walley K.R., Russell J.A. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit. Care Med. 2011;39:259–265. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 99.Wiedemann H.P., Wheeler A.P., Bernard G.R., Thompson B.T., Hayden D., Deboisblanc B., Connors A.F.J., Hite R.D., Harabin A.L. Comparison of Two Fluid-Management Strategies in Acute Lung Injury. N. Engl. J. Med. 2006;354:2564–2575. doi: 10.1016/j.jvs.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 100.Prowle J.R., Kirwan C.J., Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat. Rev. Nephrol. 2014;10:37–47. doi: 10.1038/nrneph.2013.232. [DOI] [PubMed] [Google Scholar]
- 101.Peake S.L., Delaney A., Bailey M., Bellomo R., Cameron P.A., Cooper D.J., Higgins A.M., Holdgate A., Howe B.D., Webb S.A., et al. Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 2014;371:1496–1506. doi: 10.1056/nejmoa1404380. [DOI] [PubMed] [Google Scholar]
- 102.Yealy D.M., Kellum J.A., Huang D.T., Barnato A.E., Weissfeld L.A., Pike F., Terndrup T., Wang H.E., Hou P.C., LoVecchio F., et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mouncey P.R., Osborn T.M., Power G.S., Harrison D.A., Sadique M.Z., Grieve R.D., Jahan R., Harvey S.E., Bell D., Bion J.F., et al. Trial of Early, Goal-Directed Resuscitation for Septic Shock. N. Engl. J. Med. 2015;372:1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 104.Semler M.W., Rice T.W. Sepsis Resuscitation: Fluid Choice and Dose. Clin. Chest Med. 2016;37:241–250. doi: 10.1016/j.ccm.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maitland K., Kiguli S., Opoka R.O., Engoru C., Olupot-Olupot P., Akech S.O., Nyeko R., Mtove G., Reyburn H., Lang T., et al. Mortality after Fluid Bolus in African Children with Severe Infection. N. Engl. J. Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 106.Andrews B., Muchemwa L., Kelly P., Lakhi S., Heimburger D.C., Bernard G.R. Simplified Severe Sepsis Protocol: A randomized controlled trial of modified early goal-directed therapy in Zambia. Crit. Care Med. 2014;42:2315–2324. doi: 10.1097/CCM.0000000000000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Andrews B., Semler M.W., Muchemwa L., Kelly P., Lakhi S., Heimburger D.C., Mabula C., Bwalya M., Bernard G.R. Effect of an Early Resuscitation Protocol on In-hospital Mortality Among Adults With Sepsis and Hypotension: A Randomized Clinical Trial. JAMA. 2017;318:1233–1240. doi: 10.1001/jama.2017.10913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tigabu B.M., Davari M., Kebriaeezadeh A., Mojtahedzadeh M. Fluid volume, fluid balance and patient outcome in severe sepsis and septic shock: A systematic review. J. Crit. Care. 2018;48:153–159. doi: 10.1016/j.jcrc.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 109.Lewin J., Maconochie I. Capillary refill time in adults. Emerg. Med. J. 2008;25:325–326. doi: 10.1136/emj.2007.055244. [DOI] [PubMed] [Google Scholar]
- 110.La Via L., Sanfilippo F., Continella C., Triolo T., Messina A., Robba C., Astuto M., Hernandez G., Noto A. Agreement between Capillary Refill Time measured at Finger and Earlobe sites in different positions: A pilot prospective study on healthy volunteers. BMC Anesthesiol. 2023;23:30. doi: 10.1186/s12871-022-01920-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hernández G., Ospina-Tascón G.A., Damiani L.P., Estenssoro E., Dubin A., Hurtado J., Friedman G., Castro R., Alegría L., Teboul J.-L., et al. Effect of a Resuscitation Strategy Targeting Peripheral Perfusion Status vs Serum Lactate Levels on 28-Day Mortality Among Patients with Septic Shock: The Andromeda-Shock Randomized Clinical Trial. JAMA. 2019;321:654–664. doi: 10.1001/jama.2019.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kattan E., Bakker J., Estenssoro E., Ospina-Tascón G.A., Cavalcanti A.B., De Backer D., Vieillard-Baron A., Teboul J.-L., Castro R., Hernández G. Hemodynamic phenotype-based, capillary refill time-targeted resuscitation in early septic shock: The ANDROMEDA-SHOCK-2 Randomized Clinical Trial study protocol. Rev. Bras. Ter. Intensiv. 2022;34:96–106. doi: 10.5935/0103-507X.20220004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meyhoff T.S., Hjortrup P.B., Wetterslev J., Sivapalan P., Laake J.H., Cronhjort M., Jakob S.M., Cecconi M., Nalos M., Ostermann M., et al. Restriction of Intravenous Fluid in ICU Patients with Septic Shock. N. Engl. J. Med. 2022;386:2459–2470. doi: 10.1056/NEJMoa2202707. [DOI] [PubMed] [Google Scholar]
- 114.Shapiro N.I., Douglas I.S., Brower R.G., Brown S.M., Exline M.C., Ginde A.A., Gong M.N., Grissom C.K., Hayden D., Hough C.I., et al. Early Restrictive or Liberal Fluid Management for Sepsis-Induced Hypotension. N. Engl. J. Med. 2023;388:499–510. doi: 10.1056/nejmoa2212663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reynolds P.M., Stefanos S., MacLaren R. Restrictive resuscitation in patients with sepsis and mortality: A systematic review and meta-analysis with trial sequential analysis. Pharmacotherapy. 2023;43:104–114. doi: 10.1002/phar.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Silversides J.A., Major E., Ferguson A.J., Mann E.E., McAuley D.F., Marshall J.C., Blackwood B., Fan E. Conservative fluid management or deresuscitation for patients with sepsis or acute respiratory distress syndrome following the resuscitation phase of critical illness: A systematic review and meta-analysis. Intensiv. Care Med. 2017;43:155–170. doi: 10.1007/s00134-016-4573-3. [DOI] [PubMed] [Google Scholar]
- 117.Michard F., Teboul J.-L. Predicting Fluid Responsiveness in ICU Patients: A critical analysis of the evidence. Chest. 2002;121:2000–2008. doi: 10.1378/chest.121.6.2000. [DOI] [PubMed] [Google Scholar]
- 118.Cherpanath T.G.V., Geerts B.F., Lagrand W.K., Schultz M.J., Groeneveld A.B.J. Basic concepts of fluid responsiveness. Neth. Hear. J. 2013;21:530–536. doi: 10.1007/s12471-013-0487-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Monnet X., Lai C., Teboul J.-L. How I personalize fluid therapy in septic shock? Crit. Care. 2023;27:123. doi: 10.1186/s13054-023-04363-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sevransky J.E. Dynamic Measures to Determine Volume Responsiveness: Logical, Biologically Plausible, and Unproven. Crit. Care Med. 2016;44:1923–1926. doi: 10.1097/CCM.0000000000001997. [DOI] [PubMed] [Google Scholar]
- 121.Monnet X., Marik P., Teboul J.-L. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensiv. Care Med. 2016;42:1935–1947. doi: 10.1007/s00134-015-4134-1. [DOI] [PubMed] [Google Scholar]
- 122.Douglas I.S., Alapat P.M., Corl K., Exline M.C., Forni L.G., Holder A.L., Kaufman D.A., Khan A., Levy M.M., Martin G.S., et al. Fluid Response Evaluation in Sepsis Hypotension and Shock: A Randomized Clinical Trial. Chest. 2020;158:1431–1445. doi: 10.1016/j.chest.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Keren H., Burkhoff D., Squara P. Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. Am. J. Physiol. Heart Circ. Physiol. 2007;293:H583–H589. doi: 10.1152/ajpheart.00195.2007. [DOI] [PubMed] [Google Scholar]
- 124.Raval N.Y., Squara P., Cleman M., Yalamanchili K., Winklmaier M., Burkhoff D. Multicenter Evaluation of Noninvasive Cardiac Output Measurement by Bioreactance Technique. J. Clin. Monit. Comput. 2008;22:113–119. doi: 10.1007/s10877-008-9112-5. [DOI] [PubMed] [Google Scholar]
- 125.Michard F., Boussat S., Chemla D., Anguel N., Mercat A., Lecarpentier Y., Richard C., Pinsky M.R., Teboul J.-L. Relation between Respiratory Changes in Arterial Pulse Pressure and Fluid Responsiveness in Septic Patients with Acute Circulatory Failure. Am. J. Respir. Crit. Care Med. 2000;162:134–138. doi: 10.1164/ajrccm.162.1.9903035. [DOI] [PubMed] [Google Scholar]
- 126.Marik P.E., Cavallazzi R., Vasu T., Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature. Crit. Care Med. 2009;37:2642–2647. doi: 10.1097/CCM.0b013e3181a590da. [DOI] [PubMed] [Google Scholar]
- 127.Jozwiak M., Monnet X., Teboul J.-L. Pressure Waveform Analysis. Anesth. Analg. 2018;126:1930–1933. doi: 10.1213/ANE.0000000000002527. [DOI] [PubMed] [Google Scholar]
- 128.Marik P.E., Monnet X., Teboul J.-L. Hemodynamic parameters to guide fluid therapy. Ann. Intensiv. Care. 2011;1:1. doi: 10.1186/2110-5820-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Monnet X., Marik P.E., Teboul J.-L. Prediction of fluid responsiveness: An update. Ann. Intensiv. Care. 2016;6:111. doi: 10.1186/s13613-016-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vignon P., Repessé X., Bégot E., Léger J., Jacob C., Bouferrache K., Slama M., Prat G., Vieillard-Baron A. Comparison of Echocardiographic Indices Used to Predict Fluid Responsiveness in Ventilated Patients. Am. J. Respir. Crit. Care Med. 2017;195:1022–1032. doi: 10.1164/rccm.201604-0844OC. [DOI] [PubMed] [Google Scholar]
- 131.La Via L., Astuto M., Dezio V., Muscarà L., Palella S., Zawadka M., Vignon P., Sanfilippo F. Agreement between subcostal and transhepatic longitudinal imaging of the inferior vena cava for the evaluation of fluid responsiveness: A systematic review. J. Crit. Care. 2022;71:154108. doi: 10.1016/j.jcrc.2022.154108. [DOI] [PubMed] [Google Scholar]
- 132.Monnet X., Shi R., Teboul J.-L. Prediction of fluid responsiveness. What’s new? Ann. Intensiv. Care. 2022;12:46. doi: 10.1186/s13613-022-01022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jozwiak M., Teboul J.-L., Monnet X. Extravascular lung water in critical care: Recent advances and clinical applications. Ann. Intensiv. Care. 2015;5:38. doi: 10.1186/s13613-015-0081-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bednarczyk J.M., Fridfinnson J.A., Kumar A., Blanchard L., Rabbani R., Bell D., Funk D., Turgeon A.F., Abou-Setta A.M., Zarychanski R. Incorporating Dynamic Assessment of Fluid Responsiveness into Goal-Directed Therapy: A Systematic Review and Meta-Analysis. Crit. Care Med. 2017;45:1538–1545. doi: 10.1097/CCM.0000000000002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Richard J.-C., Bayle F., Bourdin G., Leray V., Debord S., Delannoy B., Stoian A.C., Wallet F., Yonis H., Guerin C. Preload dependence indices to titrate volume expansion during septic shock: A randomized controlled trial. Crit. Care. 2015;19:5. doi: 10.1186/s13054-014-0734-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ince C., Boerma E.C., Cecconi M., De Backer D., Shapiro N.I., Duranteau J., Pinsky M.R., Artigas A., Teboul J.-L., Reiss I.K.M., et al. Second consensus on the assessment of sublingual microcirculation in critically ill patients: Results from a task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2018;44:281–299. doi: 10.1007/s00134-018-5070-7. [DOI] [PubMed] [Google Scholar]
- 137.Edul V.K., Gutierrez F.J. Devices for assessing microcirculation. Curr. Opin. Crit. Care. 2023;29:236–243. doi: 10.1097/MCC.0000000000001044. [DOI] [PubMed] [Google Scholar]
- 138.Schneider A.G., Goodwin M.D., Schelleman A., Bailey M., Johnson L., Bellomo R. Contrast-enhanced ultrasonography to evaluate changes in renal cortical microcirculation induced by noradrenaline: A pilot study. Crit. Care. 2014;18:653. doi: 10.1186/s13054-014-0653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lima A.M., van Rooij T., Ergin B., Sorelli M.M., Ince Y.M., Specht P.A.C.B., Mik E.G.M., Bocchi L., Kooiman K., de Jong N., et al. Dynamic Contrast-Enhanced Ultrasound Identifies Microcirculatory Alterations in Sepsis-Induced Acute Kidney Injury. Crit. Care Med. 2018;46:1284–1292. doi: 10.1097/CCM.0000000000003209. [DOI] [PubMed] [Google Scholar]
- 140.Mongkolpun W., Gardette M., Orbegozo D., Vincent J.-L., Creteur J. An increase in skin blood flow induced by fluid challenge is associated with an increase in oxygen consumption in patients with circulatory shock. J. Crit. Care. 2022;69:153984. doi: 10.1016/j.jcrc.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 141.Mongkolpun W., Orbegozo D., Cordeiro C.P.R., Franco C.J.C.S., Vincent J.-L.M., Creteur J. Alterations in Skin Blood Flow at the Fingertip Are Related to Mortality in Patients With Circulatory Shock. Crit. Care Med. 2020;48:443–450. doi: 10.1097/CCM.0000000000004177. [DOI] [PubMed] [Google Scholar]
- 142.Duranteau J., De Backer D., Donadello K., Shapiro N.I., Hutchings S.D., Rovas A., Legrand M., Harrois A., Ince C. The future of intensive care: The study of the microcirculation will help to guide our therapies. Crit. Care. 2023;27:190. doi: 10.1186/s13054-023-04474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.