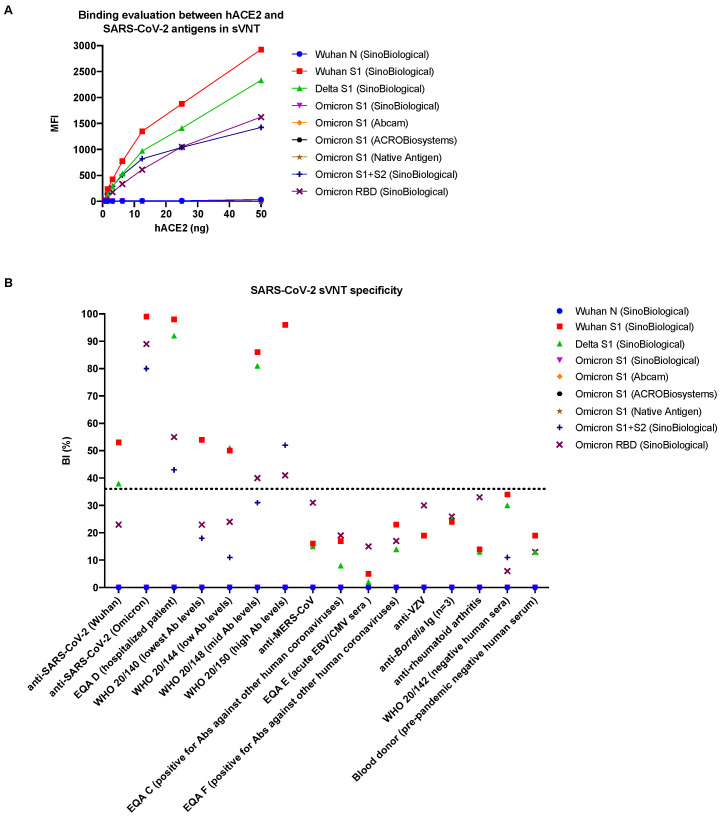
Figure 5.
Evaluation of the SARS-CoV-2 sVNT. (A) Binding between the hACE2 receptor and SARS-CoV-2 antigens in the SARS-CoV-2 sVNT. Data points represent seven different concentrations of hACE2 (0.78–50 ng, two-fold dilutions). (B) Specificity of antigens in the SARS-CoV-2 sVNT. For the anti-SARS-CoV-2 (Omicron), WHO 20/140, WHO 20/150, and anti-Varicella Zoster controls, the BI (%) was identical for the Wuhan S1 and Delta S1 antigens, resulting in overlapping data points in the plot and thereby non-visible data points for the Delta S1 antigen. sVNT—surrogate virus neutralization test; BI (%)—binding inhibition percentage; N—nucleocapsid protein; S—spike protein; S1—domain of the spike protein involved in receptor binding; S2—membrane fusion domain of the spike protein; hACE2—human angiotensin-converting enzyme 2; SARS-CoV-2—Severe acute respiratory syndrome coronavirus 2; Wuhan, Delta, Omicron—variants of SARS-CoV-2; EQA C-F—serum samples that are part of the European Centre for Disease Prevention and Control’s SARS-CoV-2 serological external quality assessment panel; WHO samples—included in the World Health Organization’s international reference panel for anti-SARS-CoV-2; MERS-CoV—Middle East respiratory syndrome coronavirus; EBV—Epstein-Barr virus; CMV—Cytomegalovirus; VZV—Varicella Zoster virus; dotted line indicates the cut-off value. The manufacturers of the antigens are presented within the parenthesis.