Table 6.
Other compounds identified in C. medica L.
| Compounds | Formula | Structure | * Extraction Method | Method Analyses | Part of the Plant | Abundance | References |
|---|---|---|---|---|---|---|---|
| Alkaloids | |||||||
| 1,2,3,4-Tetrahydro-beta-carboline-3-carboxylic acid | C12H12N2O2 |
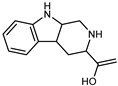
|
IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Acridine derivatives | |||||||
| Medicacridone | C20H21NO4 |
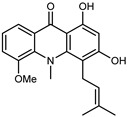
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Citracridone-I | C20H19NO5 |
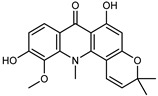
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| citracridone-III | C19H17NO5 |
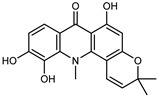
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| 5-hydroxynoracronycine 3 | C19H17NO4 |
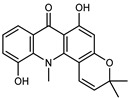
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Xanthones | |||||||
| Medicaxanthone | C51H75O8 |
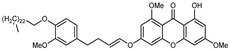
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Lichenxanthone | C15H12O6 |
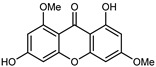
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Glycol | |||||||
| 2,3-Butanediol | C4H10O2 |
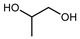
|
X | GC–MS | exocarp, mesocarp | 23.7% | [54] |
| Furan derivatives | |||||||
| Furfural | C5H4O2 |
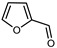
|
X | GC–MS | exocarp, mesocarp | 3.9% | [54] |
| 2(3H)-Furanone, 5-methyl | C8H12O2 |
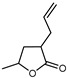
|
X | GC–MS | exocarp, mesocarp | 0.9% | [54] |
| 5- 5-Hydroxymethylfurfural | C6H6O3 |
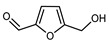
|
X | GC–MS | exocarp, mesocarp | 1.9% | [54] |
| Hydrocarbons | |||||||
| 1,3-Cyclopentadiene | C5H6 |
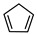
|
XIII | GC–MS | fresh fructus | 1.75–2.36% | [45] |
| Benzene | C6H6 |
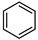
|
XI | GC–MS | fructus | 1.67% | [58] |
| Eicosane | C20H42 |
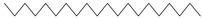
|
I | GC–MS | flavedo | 0.10% | [50] |
| Nonacosane | C29H60 |

|
I | GC–MS | flavedo | 0.10% | [50] |
| Mono or polyunsaturated aldehyde | |||||||
| Undecanal | C11H22O |
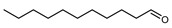
|
V, VI, VII | GC–MS | flavedo | 0.1–0.2% | [48] |
| II, III, IV | HRGC–MS | flavedo | 0.03–0.06% | [49] | |||
| Dodecanal | C12H24O |

|
II, III, IV | HRGC–MS | flavedo | 0.02–0.03% | [49] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| 9,17-octadecadienal | C18H32O |
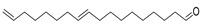
|
I | GC–MS | flavedo | 9.29% | [50] |
| 16-Octadecenal | C18H34O |

|
I | GC–MS | flavedo | 0.10% | [50] |
| Nonanal | C9H18O |
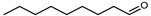
|
II, III, IV | HRGC–MS | flavedo | 0.04–0.07% | [49] |
| Tetradecanal | C14H28O |

|
V, VI, VII | GC–MS | flavedo | 0.1% | [48] |
| Pentadecanal | C15H30O |

|
V, VI, VII | GC–MS | flavedo | 0.1% | [48] |
| Phenylpropanoids | |||||||
| Coniferin | C16H22O8 |
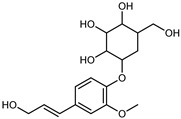
|
IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Syringin | C17H24O9 |
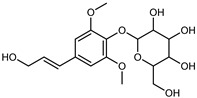
|
IX | EI–MS, HR–EI-MS | fresh fruit | - | [43] |
| Phytosterols | |||||||
| Lupeol | C26H32O7 |
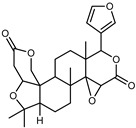
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Stigmasterol | C29H48O |
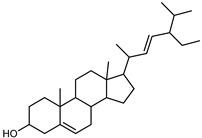
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Ꞵ-Sitosterol | C29H50O |
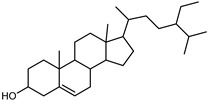
|
VIII | ESI–HR, EI–MS, HMQC, HMBC | bark | - | [37] |
| Fatty acids and their esters | |||||||
| Lauric acid | C12H24O2 |
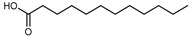
|
I | GC–MS | flavedo | 0.11% | [50] |
| Myristic acid | C14H28O2 |
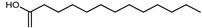
|
I | GC–MS | flavedo | 0.23% | [50] |
| Palmitic acid | C16H32O2 |
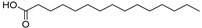
|
I | GC–MS | flavedo | 5.17% | [50] |
| Hexadecanal | C16H32O |

|
XIII | GC–MS | mesocarp | 1.6% | [54] |
| V, VI, VII | GC–MS | flavedo | 0.1% | [48] | |||
| Pentadecanoic acid methyl ester | C16H32O2 |
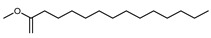
|
I | GC–MS | flavedo | 0.22% | [50] |
| Palmitoleic acid | C17H32O2 |
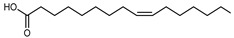
|
I | GC–MS | flavedo | 0.19% | [50] |
| Heptadecanoic acid | C17H34O2 |
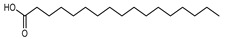
|
I | GC–MS | flavedo | 0.20% | [50] |
| Stearic acid | C18H36O2 |
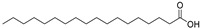
|
I | GC–MS | flavedo | 0.18% | [50] |
| 16-Octadecenal | C18H34O |

|
I | GC–MS | flavedo | 0.10% | [50] |
| Linoleic acid, methyl ester | C19H34O2 |
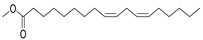
|
I | GC–MS | flavedo | 0.19% | [50] |
| Linolenic acid, methyl ester | C19H32O2 |
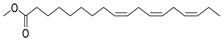
|
I | GC–MS | flavedo | 0.41%0.30% | [50] |
| Stearic acid, methyl ester | C19H38O2 |
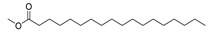
|
I | GC–MS | flavedo | 0.30% | [50] |
| Benzoates | |||||||
| Methyl vanillate methyl ester |
C9H10O4 |
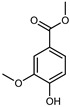
|
IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Methyl benzoate | C8H8O2 |
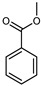
|
IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
| Methyl paraben | C8H8O3 |
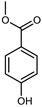
|
IX | EI–MS, HR–EI–MS | fresh fruit | - | [43] |
* Extraction method. I: Maceration of peel with n-hexane at room temperature; II: aspiration of the oil from the utricles present on the peel by means of a syringe with a thin needle; III: the rinds of the fruit were squeezed to cause the breaking of the utricles in order to release the oil, which was collected by extraction with hexane; IV: manual abrasion of the rind by means of a stainless steel grater, followed by manual pressing and centrifugation of the water–oil emulsion; V: hydro-distillation; VI: soxhlet apparatus using pentane and ethanol as solvents; VII: SCF–CO2; VIII: maceration in MeOH; IX: MeOH under reflux; X: maceration and UAE in MeOH; XI: distillation using a Clevenger-type apparatus; XII: steam-based hydro-distillation; XIII: exocarp and mesocarp were pulverized in liquid nitrogen with a chilled mortar and pestle, and then weighed and placed in MeOH. The mixtures were sonicated.
