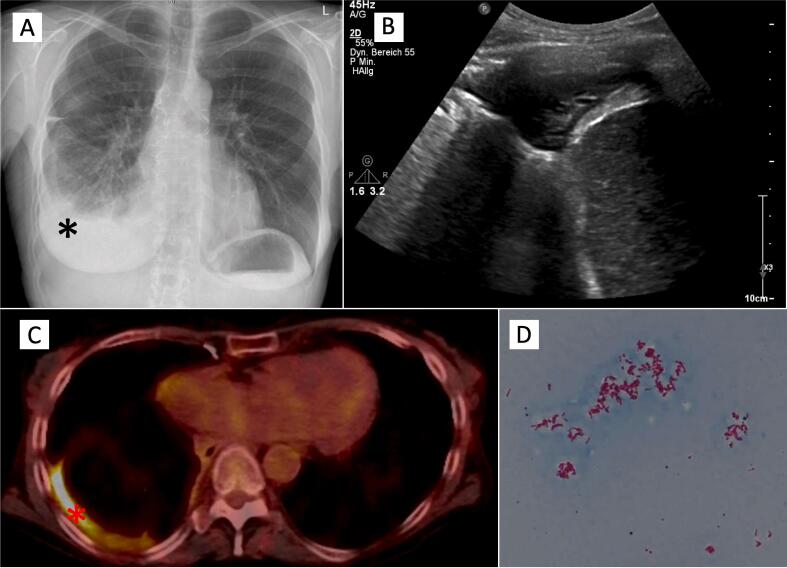
A 62-year-old woman with history of re-do bilateral-lung transplantation in 2012 and 2020 was hospitalized in May 2022 for fever of up to 38,6 °C for 4 weeks, night sweat, declining graft function and right sided chest pain. Primary lung transplantation was performed in 2012 for bronchiectasis without evidence of chronic bacterial colonization and secondary lung transplantation for chronic lung allograft dysfunction without evidence of chronic bacterial colonization. Forced vital capacity (FVC) and forced expiratory volume (FEV1) declined to 72% and 78% of baseline at presentation. Laboratory test results at the day of admission are shown in Table 1. On examination, the temperature was 38.2 °C, the pulse 80 beats per minute, blood pressure 110/95 mmHg and oxygen saturation 98% while the patient was breathing ambient air. Chest auscultation revealed normal breath sounds and percussion revealed dull sounds on the right basal chest wall. Four weeks earlier as part of a visit to our lung transplant outpatient clinic, a bronchoscopy was performed due to declining lung function as measured by the monitor. Acid-fast organisms were detected on direct smear from bronchoalveolar lavage (BAL) fluid. However, TBC-PCR remained negative and transbronchial biopsy demonstrated no sign of acute cellular rejection (A B Grade). Furthermore, cultures were taken to screen for NTM-TBC and Nocardia. Chest X-ray revealed a new right side pleural effusion (see Fig. 1A), which was confirmed by chest computed tomography. A diagnostic puncture of pleural fluid was performed. On thoracic ultrasound a small fluid collection with consolidated pleural tissue, no drainage or laboratory analysis of the fluid was possible due to minimal sample volume. Initial bacterial cultures of pleural fluids were negative.
Table 1.
Microbiological, laboratory and pulmonary parameters of lung transplant patient.
| At hospitalitsation | 3 months after hospitalisation | 6 months after hospitalisation | |
|---|---|---|---|
| Blood culture | Negative | Negative | Negative |
| BAL | |||
| culture | Mycobacterium kansasii | No evidence of mycobacteria | Not performed |
| AFB stain | positive | negative | |
| Pleural punctate | |||
| Culture | Mycobacterium kansasii | Not performed | Not performed |
| AFB stain | positive | ||
| EBV DNA-PCR | < 3000 IU/ml | Not performed | Not performed |
| Laboratory tests | |||
| CRP, mg/l (normal <5) | 96,8 | 65,4 | 6,5 |
| Leukocytes, Tsd/µl (normal 2.9 - 10.02) | 3,6 | 3,1 | 4 |
| PCT, µg/l (normal < 0.5) | 0,2 | 0,1 | 179 |
| Creatinine, µmol/l (normal < 84) | 154 | 202 | |
| LDH, U/l (normal < 247) | 328 | 199 | |
| Ferritin, µmol/l (normal <365) | 3747 | 1754 | |
| Temperature, °C | 38,5 | 35,4 | 37 |
| Blood gas analysis | No supplemental Oxygen | No supplemental Oxygen | No supplemental Oxygen |
| pO2, mmHg | 93 | 84 | 90 |
| pCO2, mmHg | 30 | 31 | 34 |
| H | 7,41 | 7,44 | 7,44 |
| HCO3, mmol/l | 20 | 22 | 24 |
| SpO2, % | 98 | 97 | 97 |
| Pulmonary function tests | |||
| FEV1, ml | 2410 (94% predicted) | 2240 (86% predicted) | 2440 (94% predicted) |
| VC, ml | 2670 (85% predicted) | 2350 (73% predicted) | 2620 (83% predicted) |
BAL = bronchoalveolar lavage; AFB = acid-fast bacilli; CRP = C-reactive protein; PCT = pro-calcitonin; pO2 = partial pressure of oxygen; pCO2 = partial pressure of carbon dioxide; HCO3 = hydrogen carbonate; SpO2 = saturation, of peripheral oxygen; FEV1 = forced expiratory volume in one second; VC = vital capacity.
Fig. 1.
Chest X-ray in p.a. with rounding of the right costodiaphragmatic recess (black star), highly suspicious for a pleural effusion (A), organized pleural effusion in the right costodiaphragmatic recess in thorax sonography (B), FDG-PET with increased metabolism along the partly nodular thickened right pleura (red star) with punctum maximum basally (C), Microscopy of M. kansasii culture isolates (acid fast stain) (D). Images courtesy of Hannover Medical School, Department of Radiology and Department of Microbiology and Hygiene.
Differential diagnoses of fever of unknown origin after transplantation includes infectious and non-infectious causes. Extensive sampling for microbiological and fungal cultures including blood, urine, stool samples and viral testing was performed and empirical antibiotic therapy with Ampicillin/Sulbactam was initiated. Epstein-Barr virus (EBV) with low viral load was detected in whole blood. In conjunction with such as fever and weight loss and cytopenia (see Table 1) there was suspicion of post-transplant lymphoproliferative disorder (PTLD). Histopathology of bone marrow was normal.
PTLD is a frequent neoplasm after solid organ transplantation [1]. A panendoscopic examination of the ear, nose and throat and an abdominal sonography exhibited no pathologically altered lymph nodes or evidence for a PTLD. An FDG-PET showed an increased metabolism along the partly nodular thickened right pleura with punctum maximum basally (see picture 1C). Furthermore, computed tomography showed no evidence of a neoplasm or pathological lymph nodes. In correlation with the clinical indications, the diagnostic findings were highly suspicious for a pleural empyema. A re-sampling of the pleural effusion (see picture 1B) confirmed microscopically acid-fast organisms, further identified as Mycobacterium kansasii by culture and sequence analysis. Cultures for Mycobacterium kansasii were positive in BAL as well. Video-assisted thoracoscopic surgery (VATS) to gain pleural tissue for diagnostic reasons was discussed extensively. Because we could already identify M. kansasii in the pleural effusion, VATS and pleural tissue sampling had no added value after risk–benefit evaluation. There are only a few known cases in which NTM infection also involves the pleura via as yet unknown mechanisms of spread [2]. In this patient, this may have occurred in the course of the re-transplantation two years ago or through another injury to the visceral pleura, e.g. in the course of a previous pneumonia. Ultimately, there is no evidence to support these hypotheses and thus the exact genesis remains speculative.
Pleural infection due to Mycobacterium kansasii is a rare condition. Twenty-three cases have been reported of this non-tuberculous mycobacterial lung disease (NTM-LD), three cases occurred after solid organ transplantation (two patients after renal transplantation and one patient after lung transplantation) [3], [4], [5].
A multicentre study found Mycobacterium kansasii to be the sixth most frequently isolated NTM from pulmonary samples both worldwide and in Europe [6]. When derived from respiratory isolates, criteria for NTM-LD by the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) must be met in order to distinguish colonisation or contaminant from a true pathogen [7]. Pulmonary infections with Mycobacterium kansasii are usually associated with immunosuppression including patients after transplantation, e. g. malignancies, HIV or underlying lung disease [3]. NTM-LD should be diagnosed when NTM-positive patients show pulmonary or systemic symptoms (e.g., dyspnoea, fever, night sweats, weight loss) which correlate with radiographic features on chest radiograph or high-resolution computed tomography (HR-CT) [7], [8]. Typical radiological findings of pulmonary disease with Mycobacterium kansasii in descending frequency are pulmonary opacities including interstitial pattern/fibrosis, cavities, nodules, bronchiectasis, pleura effusion, mediastinal lymphadenopathy [9]. Given the fact that both from BAL and from pleural effusion sample Mycobacterium kansasii was cultured and the diagnostic exclusion of lymphoma we diagnosed an atypical mycobacterial infection with Mycobacterium kansasii.
The current guidelines recommend a regimen of rifampicin, ethambutol, and either isoniazid or macrolid. We initiated a triple therapy with ethambutol, rifampicin and azithromycin [10]. Mycobacterium kansasii proved to be rifampicin- and macrolide-susceptible based on a resistance profile performed by the German National reference centre for mycobacteria. As described in studies before, induction of CYP3A isoenzymes in the liver and intestinal wall by rifampicin caused decreased serum levels of tacrolimus [11], [12]. Tacrolimus doses after initiation of NTM therapy were quintuplicated to reach target levels. The dosage of prednisolone was increased temporarily in order to prevent allograft rejection and immune reconstitution inflammatory syndrome (IRIS) [13]. The laboratory results improved, the fever disappeared after two weeks, chest pain resolved and pulmonary function tests showed stable parameters. NTM therapy was tolerated with minor nausea and an additional weight loss of 2 kg during the following 4 weeks.
In a 3-month follow-up visit in our outpatient clinic for lung transplantation the patient was afebrile with improved exercise capacity. Physical activity had been resumed and fatigue improved. Laboratory results showed decreased inflammation parameters (Table 1) as well as no signs of side effects due to the therapy. BAL and sputum revealed no evidence for Mycobacterium kansasii. In a 6-month follow-up visit in our outpatient clinic pleural effusion showed regression and transplant function returned to baseline values. Further, both follow-up visits included evaluation of side effects in relation to the therapy.
Ethics approval and consent to participate
Approval of the local ethic committee was not necessary. The patient provided written informed consent allowing the use of her data for scientific research.
Consent to participate
The patient provided written informed consent allowing the use of their data for scientific research.
Consent for publication
Not applicable.
Funding
Not applicable.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Pia Maria Plank reports no conflict of interest. Christopher Alexander Hinze reports no conflict of interest. Sedlacek Ludwig reports no conflict of interest. Tobias Welte T.W. and/or his institution received grants advisory/lecture/clinical trial fees and non– financial support by DFG (German Research Council), BMBF (German Ministry of Research and Education), BMG (German Ministry of Health), EU, WHO, AstraZeneca, Basilea, Biotest, Bayer, Boehringer, Berlin Chemie, GSK, Infectopharm, MSD, Novartis, Pfizer, Roche, AstraZeneca, Basilea, Biotest, Bayer, Boehringer, Gilead, GSK, Janssen, Novartis, Pfizer, Roche, all outside the submitted work. Hendrik Suhling reports personal fees/speaker honoraria from Astrazeneca, GSK, Novartis, Sanofi, outside the submitted work. Jens Gottlieb reports institutional research grants from Zambon /Breath Therapeutics, German Center of Lung Research, Deutsche Forschungsgemeinschaft. He also received fees for advisory/consultancy from Theravance, Pierre Fabre, Precision, Atheneum, Merck, Springer Healthcare, European Research Network and speaker fees from Novartis, Astra Zeneca.
References
- 1.Allen U.D., Preiksaitis J.K. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant. 2019;33(9) doi: 10.1111/ctr.13652. [DOI] [PubMed] [Google Scholar]
- 2.Park S., et al. Clinical characteristics and treatment outcomes of pleural effusions in patients with nontuberculous mycobacterial disease. Respir Med. 2017;133:36–41. doi: 10.1016/j.rmed.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Luttmann K.F., et al. Pleural effusion/empyema with Mycobacterium kansasii. Cureus. 2022;14(1):e21300. doi: 10.7759/cureus.21300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paull, D.E.D., Gary R.; Brown, Robert L., Mycobacterium kansasii empyema in a renal transplant recipient case report and review of the literature. 2003. 76(1): p. 270-271. [DOI] [PubMed]
- 5.Cruz N., et al. Pulmonary infection with M. kansasii in a renal transplant patient. Nephron. 1980;26(4):187–188. doi: 10.1159/000181978. [DOI] [PubMed] [Google Scholar]
- 6.Hoefsloot W., et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42(6):1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 7.Kurz S.G., et al. Summary for clinicians: 2020 clinical practice guideline summary for the treatment of nontuberculous mycobacterial pulmonary disease. Ann Am Thorac Soc. 2020;17(9):1033–1039. doi: 10.1513/AnnalsATS.202003-222CME. [DOI] [PubMed] [Google Scholar]
- 8.Moon S.M., et al. Clinical significance of Mycobacterium kansasii isolates from respiratory specimens. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0139621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakuła Z., et al. Clinical, radiological and molecular features of Mycobacterium kansasii pulmonary disease. Respir Med. 2018;139:91–100. doi: 10.1016/j.rmed.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Daley C.L., et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin Infect Dis. 2020;71(4):e1–e36. doi: 10.1093/cid/ciaa241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staatz C.E., Tett S.E. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623–653. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 12.Bhaloo S., Prasad G.V. Severe reduction in tacrolimus levels with rifampin despite multiple cytochrome P450 inhibitors: a case report. Transplant Proc. 2003;35(7):2449–2451. doi: 10.1016/j.transproceed.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura S., et al. Hemophagocytic syndrome-like tuberculosis-immune reconstitution inflammatory syndrome after the initiation of hepatic tuberculosis treatment. Cureus. 2022;14(5) doi: 10.7759/cureus.24644. [DOI] [PMC free article] [PubMed] [Google Scholar]