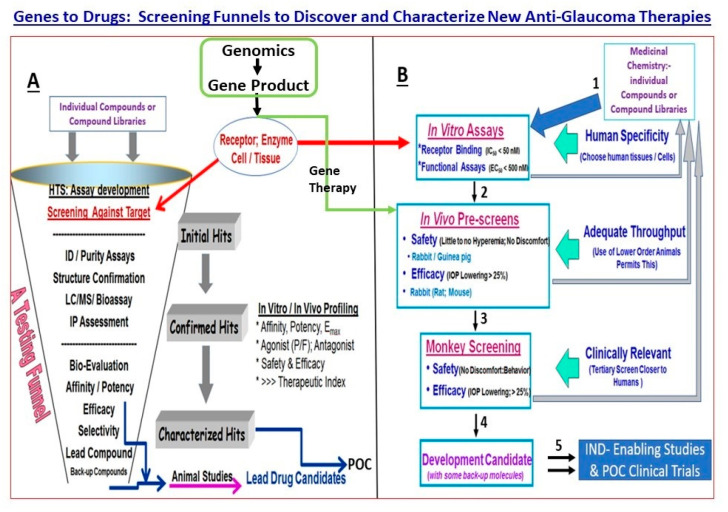
Figure 3.
Displayed in this figure are a typical testing funnel and the components therein for synthesis of test compounds and/or gene therapy capsids and their characterization, followed by their evaluation in vitro and in vivo to eventually produce suitable compounds for progression towards Investigational New Drug (IND)-enabling studies (A). A more comprehensive screening paradigm for discovering and analyzing the pharmacological and biochemical features of test agents in a variety of in vitro assay systems, followed by screening in animal models of increasing complexity, is shown in (B). Examples of certain stage-gate passing criteria for progression down the testing funnel are also included for illustration purposes. Once the preferred compound/gene therapy has met all the prescribed criteria, it can undergo IND-enabling studies and then into proof-of-concept clinical trials for the target disease (e.g., for elevated IOP; slowing RGC/optic nerve damage). Figures are adapted from [15,16], the author’s own recent publications.