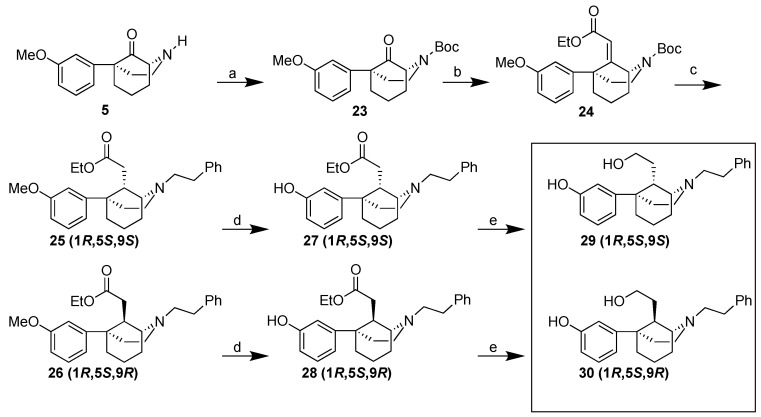
Scheme 4.
Synthesis of 1R,5S,9S- and 1R,5S,9R-C9-hydroxyethyl-5-phenylmorphan diastereomers 29 and 30. Reagents and conditions: (a) Boc2O, triethylamine, DMAP, DCM, 78%; (b) NaH, triethyl phosphonoacetate, THF, reflux, 16 h, 95%; (c) i. Escat 103 (5% Pd/C), 60 °C, 50 psi, 1 h, ii. triflouroacetic acid, DCM, 0 °C, 1 h, iii. Ph(CH2)2Br, K2CO3, MeCN, reflux 18 h, 80% over 3 steps, 1:3 25 (1R,5S,9S):26 (1R,5S,9R); (d) BBr3, DCM, −78 °C→rt, 1 h, 98% (e) LiAlH4, THF, 0 °C, 1 h, 47% 29 (1R,5S,9R), 66% 30 (1R,5S,9S).