Figure 7.
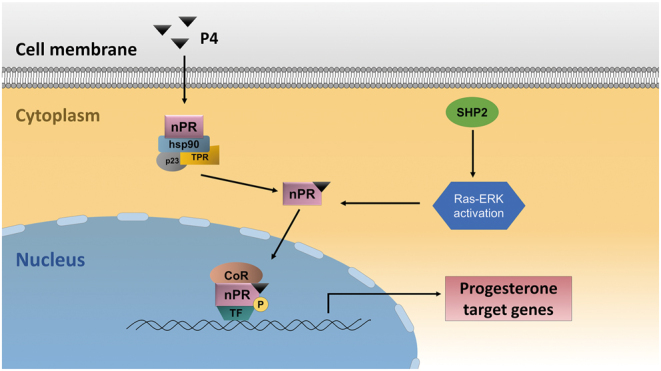
Illustrative model of uterine SHP2 maintaining PR function during decidualization. In the absence of progesterone ligands, PR and chaperone complexes form inhibitory proteins, including heat shock protein 90 (hsp90), p23 and cochaperones containing tetratricopeptide repeats (TPRs). The binding of progesterone activates PR by inducing conformational changes, phosphorylation, nuclear translocation, and recruitment of a series of coactivators (CoA) to regulate target gene transcription. SHP2 participates in decidualization by activating Ras–ERK to maintain the normal nuclear localization of PR and the expression of downstream target genes. CoR, coregulator; P4, progesterone; PR, progesterone receptor; SHP2, Src homology-2 domain-containing protein tyrosine phosphatase-2; TF, transcription factor.

 This work is licensed under a
This work is licensed under a