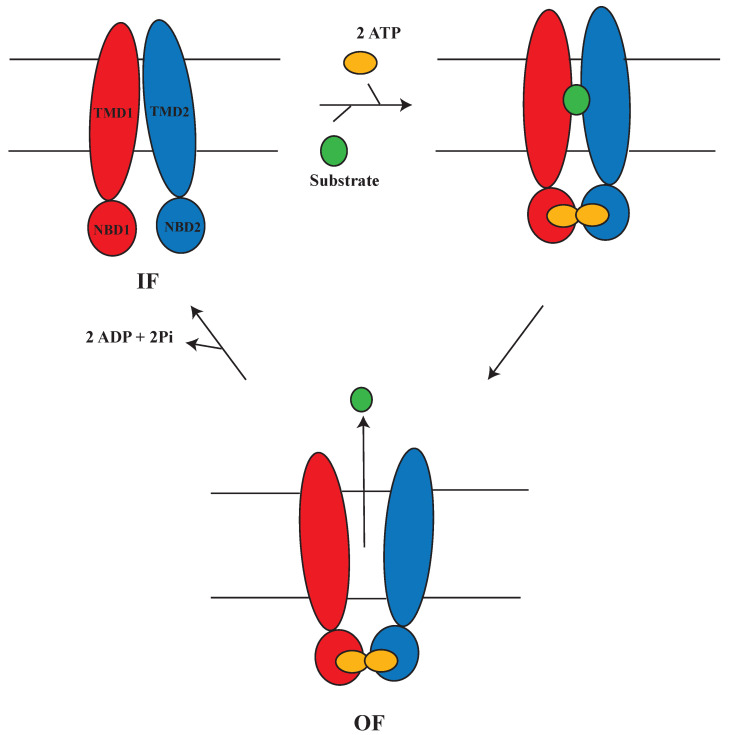
Figure 3.
Schematic representation of the alternating access mechanism in ATP-binding (ABC) transporters. The substrate (green circle) initiates the transport cycle by binding to a substrate binding site formed by the TMDs. The NBDs then undergo a conformational change that permits ATP binding (orange ellipse) and the formation of a closed NBD dimer. A significant conformational change in the TMDs is triggered by the closed NBD dimer, and TMDs open toward the outside, allowing substrate translocation to begin. Dissociation of NBD dimer is triggered by ATP hydrolysis, and the transporter returns to an inward-facing conformation state by releasing phosphate and ADP.