Abstract
Purpose
The incidence and prognostic implications of atrial fibrillation (AF) in patients with severe aortic stenosis (AS) undergoing transcatheter aortic valve implantation (TAVI) are controversial, especially for Korean patients. Furthermore, the pattern of antithrombotic therapy for these patients is unknown. The present study sought to identify the impact of AF on Korean patients undergoing TAVI and demonstrate the status of antithrombotic therapy for these patients.
Materials and Methods
A total of 660 patients who underwent TAVI for severe AS were recruited from the nationwide K-TAVI registry in Korea. The enrolled patients were stratified into sinus rhythm (SR) and AF groups. The primary endpoint was all-cause death at 1-year.
Results
AF was recorded in 135 patients [pre-existing AF 108 (16.4%) and new-onset AF 27 (4.1%)]. The rate of all-cause death at 1 year was significantly higher in patients with AF than in those with SR [16.2% vs. 6.4%, adjusted hazard ratio (HR): 2.207, 95% confidence interval (CI): 1.182–4.120, p=0.013], regardless of the onset timing of AF. The rate of new pacemaker insertion at 1 year was also significantly higher in patients with AF than in those with SR (14.0% vs. 5.5%, adjusted HR: 3.137, 95%CI: 1.621–6.071, p=0.001). Among AF patients, substantial number of patients received the combination of multiple antithrombotic agents (77.8%), and the most common combination was that of aspirin and clopidogrel (38.1%).
Conclusion
AF was an independent predictor of 1-year mortality and new pacemaker insertion in Korean patients undergoing TAVI.
Keywords: Transcatheter aortic valve implantation, atrial fibrillation, Korean, antithrombotic agent
Graphical Abstract
INTRODUCTION
Transcatheter aortic valve implantation (TAVI) has developed enormously over the past decade and become a standard of care for patients with symptomatic severe aortic stenosis (AS).1,2 Atrial fibrillation (AF) is a common complication following TAVI or surgical aortic valve replacement in patients with severe AS,3,4,5,6 since AF and AS share similar cardiovascular risk factors, and pressure overload on the left heart system due to AS is related to a higher incidence of AF. Furthermore, the TAVI procedure itself could be associated with a systemic inflammatory response and oxidative stress, which result in slow atrial conduction, short refractoriness, and endo-epicardial dissociation that can in turn induce re-entry, ectopic activity, and new-onset AF.7 Theoretically, the atrioventricular desynchrony and irregular ventricular contraction caused by AF could impair the heart function of patients with severe AS. In this regard, AF has been associated with poorer clinical outcomes than those found in patients with a sinus rhythm (SR) after TAVI.3,4,7,8,9,10,11,12,13 However, evidence is lacking about the incidence and prognostic implications of AF in Korean patients with AS undergoing TAVI.
Although the current guidelines recommend the use of an oral anticoagulation (OAC) with or without antiplatelet therapy after TAVI in patients who require long-term anticoagulation, the optimal antithrombotic regimens for AF patients with AS undergoing TAVI are still unclear.1,14 Thus, the management of antithrombotic therapy is frequently individualized to balance the prevention of ischemic events and the risk of life-threatening bleeding, which has translated into wide variability in treatment across centers and operators.15 Therefore, we used a nationwide multicenter registry from Korea to identify the prognostic implications of AF on Korean patients and find the current patterns of post-TAVI antithrombotic regimen.
MATERIALS AND METHODS
Study population and data collection
The second cohort of the Korean-Transcatheter Aortic Valve Implantation (K-TAVI) registry contains retrospective, nationwide, and observational data from consecutive patients who underwent TAVI at 21 participating centers in Korea from June 2015 to June 2018 (Supplementary Fig. 1, only online). Clinical information about patient demographics, comorbidities, functional status, hemodynamics, procedural details, and 1-year clinical outcomes were collected using uniform, web-based case report forms. Data quality checks were implemented at the steering committee meeting of the K-TAVI registry. If patients were lost to follow-up, the attending physician contacted the patient or their family members by telephone. The present study was performed in compliance with the Declaration of Helsinki. This study was approved by the institutional review board of Samsung Medical Center (IRB number: 2018-12097), and informed consent from individual patients was waived by the institutional review board at each center.
Patient selection and procedures
The diagnosis of severe AS and eligibility for TAVI were adjudicated by each center’s local multidisciplinary team consisting of interventional cardiologists, cardiac surgeons, imaging cardiologists, radiologists, and anesthesiologists. TAVI was performed mainly through a transfemoral approach with the self-expandable Medtronic CoreValve or CoreValve Evolut R (Medtronic, Minneapolis, MN, USA) and the balloon-expandable Sapien XT and Sapien 3 transcatheter heart valves (Edwards LifeSciences, Irvine, CA, USA). Following the procedure, patients were admitted to the intensive care unit and monitored for hemodynamic and electrocardiographic status. In the general ward, all rhythm disturbances were recorded continuously by electrocardiographic telemetry. Unless contraindicated, aspirin (100 mg) was prescribed indefinitely, and clopidogrel (75 mg) was prescribed for at least 6 months after the procedure. In patients with AF, anticoagulation with a vitamin K antagonist or non-vitamin K antagonist oral anticoagulant (NOAC) was considered based on the current guideline.16
Study definitions and outcomes
The primary endpoint of this study was all-cause death at 1 year after TAVI. Secondary endpoints were changes in the New York Heart Association (NYHA) classification, changes in echocardiographic parameters, cardiac death at 1 year, stroke at 1 year, bleeding at 1 year, and pacemaker insertion at 1 year. Cardiac deaths included all deaths without a definite non-cardiac cause. Stroke included all ischemic stroke and hemorrhagic stroke regardless of onset time, but not transient ischemic attacks. Bleeding included all types of bleeding, including major and minor bleeding. All endpoints were defined according to the definitions of the Valve Academic Research Consortium-2.17
For the purpose of this study, AF patients were categorized into two groups: 1) pre-existing AF, diagnosed before TAVI; and 2) new-onset AF, diagnosed within 30 days after TAVI in patients without evidence of previous AF. Patients with atrial flutter, which can coexist with or precede AF, were assigned to the AF group.
Statistical analysis
Continuous variables are presented as the mean±standard deviation, and the differences between variables were evaluated using Welch’s t-test. Categorical variables are presented as frequencies and percentages, and were analyzed using the chi-square test and Fisher’s exact test, as appropriate. The cumulative incidence of events is described using Kaplan-Meier estimates, and was compared using the log-rank test. To calculate hazard ratios (HR) with 95% confidence intervals (CI) for the risk of clinical events, Cox proportional hazards model was used. The multivariable model contained covariates that were significant in the univariate analysis or clinically important. The Cox regressions to identify independent predictors of clinical events were based on age, sex, hypertension, diabetes mellitus, previous history of stroke, previous myocardial infarction, previous cardiac surgery, left bundle branch block, Society of Thoracic Surgeons (STS) score, Euro-score II, CHAS2-VaSc score, combined coronary artery disease, aortic valve peak jet velocity, moderate or severe aortic regurgitation, moderate or severe mitral regurgitation, left ventricular ejection fraction (LVEF), and the NYHA classification. A multivariable logistic regression analysis was performed to evaluate the independent predictors of new-onset AF after TAVI based on age, sex, body mass index, previous cardiac surgery, LVEF, left atrial volume index, E/e’, device size, anesthesia duration, pre-TAVI creatinine, and CHAS2-VaSc score. All probability values were two-sided, and p-values<0.05 were considered statistically significant. Statistical analyses were performed using R Statistical Software (version 4.0.2; R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Baseline clinical and procedural characteristics
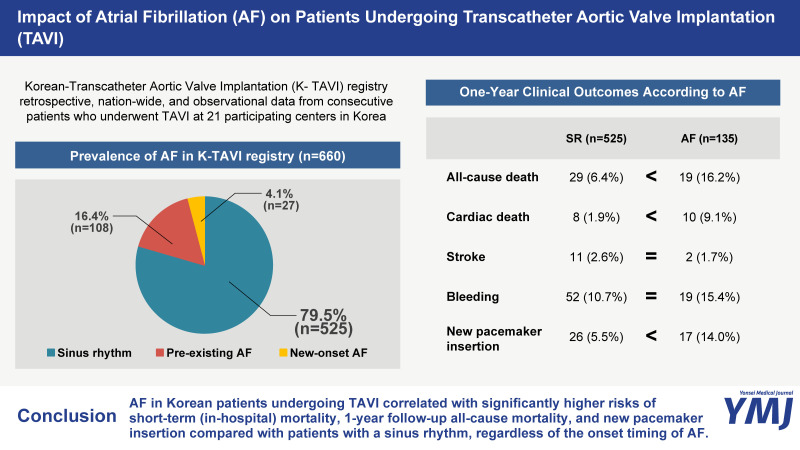
Among the total 660 patients who underwent TAVI, 135 (20.5%) patients had AF. Among the AF patients, 108 (16.4%) patients were diagnosed before TAVI, and 27 (4.1%) patients acquired new-onset AF within 30 days after TAVI (Supplementary Fig. 1, only online).
Patients with AF had significantly more previous history of stroke, but none of the other cardiovascular risk factors differed significantly between the SR and AF groups. The STS score, Euro-score II, and CHAS2-VaSc score were significantly higher in the AF group than in the SR group. Procedural characteristics, such as the rate of successful implantation, puncture to close time, and device size, did not differ between the two groups. Patients with a SR were prescribed more antiplatelet drugs (aspirin and clopidogrel), and patients with AF received more anticoagulation therapy with a vitamin K antagonist or NOAC (Table 1). Supplementary Tables 1 and 2 (only online) shows the baseline clinical and procedural characteristics of patients with pre-existing AF and new-onset AF, none of which differed between the groups except anesthesia time, which was longer in the new-onset AF group.
Table 1. Baseline Clinical and Procedural Characteristics According to AF.
| Variables | SR (n=525) | AF (n=135) | p value | ||
|---|---|---|---|---|---|
| Demographics | |||||
| Age, yr | 78.3±6.6 | 79.5±6.8 | 0.083 | ||
| Sex, male | 261 (49.7) | 67 (49.6) | >0.999 | ||
| Body mass index, kg/m2 | 23.9±3.7 | 23.7±3.3 | 0.526 | ||
| Risk factors | |||||
| Hypertension | 387 (73.7) | 104 (77.0) | 0.498 | ||
| Diabetes mellitus | 182 (34.7) | 51 (37.8) | 0.566 | ||
| Previous history of stroke | 47 (9.0) | 28 (20.7) | 0.008 | ||
| Peripheral artery disease | 74 (14.1) | 14 (10.4) | 0.320 | ||
| Previous myocardial infarction | 47 (9.0) | 14 (10.4) | 0.733 | ||
| Previous percutaneous coronary intervention | 141 (26.9) | 31 (23.0) | 0.418 | ||
| COPD | 74 (14.1) | 18 (13.3) | 0.929 | ||
| Current smoking | 22 (4.2) | 8 (5.9) | 0.528 | ||
| Previous AVR | 9 (1.7) | 2 (1.5) | >0.999 | ||
| Previous CABG | 18 (3.4) | 2 (1.5) | 0.370 | ||
| Previous renal replacement therapy | 37 (7.0) | 6 (4.4) | 0.369 | ||
| ECG findings | |||||
| Rhythm | <0.001 | ||||
| Sinus rhythm | 525 (100) | 0 (0) | |||
| Pre-existing AF or flutter | 0 (0) | 108 (80.0) | |||
| New onset AF or flutter | 0 (0) | 27 (20.0) | |||
| LBBB | 12 (2.3) | 3 (2.2) | >0.999 | ||
| RBBB | 36 (6.9) | 15 (11.1) | 0.142 | ||
| Pacing rhythm | 11 (2.1) | 1 (0.7) | 0.491 | ||
| Risk scoring systems | |||||
| STS score | 7.2±6.5 | 8.8±7.2 | 0.013 | ||
| Euro-score II | 10.2±8.5 | 12.1±9.5 | 0.024 | ||
| CHAS2-VaSc score | 4.0±1.3 | 4.3±1.5 | 0.017 | ||
| Anatomical characteristics | |||||
| AV morphology | 0.636 | ||||
| Tricuspid | 464 (88.4) | 123 (91.1) | |||
| Bicuspid | 56 (10.6) | 12 (8.9) | |||
| Other | 5 (1.0) | 0 (0) | |||
| Combined CAD | 205 (43.7) | 78 (40.6) | 0.521 | ||
| Left main or proximal LAD involvement | 107 (20.4) | 26 (19.3) | 0.865 | ||
| Annulus diameter, mm | 22.8±2.7 | 23.1±2.7 | 0.253 | ||
| Laboratory findings | |||||
| Creatinine, mg/dL | 1.4±1.6 | 1.3±1.2 | 0.316 | ||
| Hematocrit, % | 34.8±5.0 | 35.0±5.3 | 0.750 | ||
| WBC, /mm3 | 7080±2961 | 6946±2653 | 0.639 | ||
| Procedural characteristics | |||||
| Successful implantation | 521 (99.2) | 134 (99.3) | >0.999 | ||
| Puncture to close time, minute | 79.8±42.5 | 80.0±38.3 | 0.964 | ||
| General anesthesia | 504 (96.0) | 129 (95.6) | >0.999 | ||
| Anesthesia time, minute | 131.3±44.7 | 132.4±46.8 | 0.797 | ||
| Transfemoral approach | 523 (99.6) | 133 (98.5) | 0.122 | ||
| Type of device | 0.212 | ||||
| SAPIEN XT or 3 | 284 (54.1) | 73 (54.1) | |||
| CoreValve or Evolut R | 216 (41.1) | 60 (44.4) | |||
| Other | 25 (4.8) | 2 (1.5) | |||
| Device size, mm | 26.0±2.4 | 26.5±2.3 | 0.053 | ||
| Medications at discharge | |||||
| Aspirin | 464 (88.4) | 81 (60.0) | <0.001 | ||
| P2Y12 inhibitor | 470 (89.5) | 99 (73.3) | <0.001 | ||
| NOAC | 33 (6.3) | 52 (38.5) | <0.001 | ||
| Warfarin | 14 (2.7) | 17 (12.6) | <0.001 | ||
| Cilostazol | 5 (1.0) | 1 (0.7) | >0.999 | ||
AF, atrial fibrillation; AV, aortic valve; AVR, aortic valve replacement; CABG, coronary artery bypass graft; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; LAD, left anterior descending artery; LBBB, left bundle branch block; NOAC, non-vitamin K antagonist oral anticoagulant; RBBB, right bundle branch block; SR, sinus rhythm; STS, Society of Thoracic Surgeons; WBC, white blood cell.
Data are presented as mean±standard deviation or n (%).
Changes in symptoms after TAVI
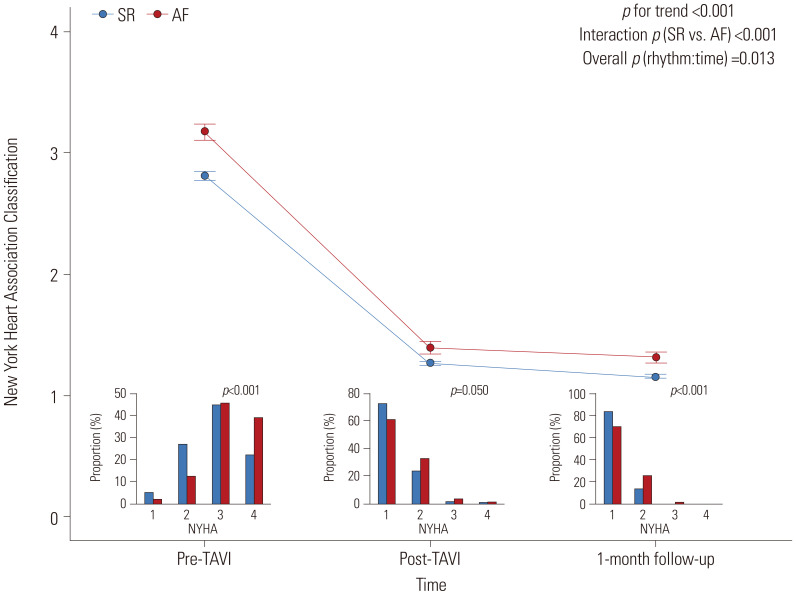
The changes in symptoms were assessed using the NYHA classification before, after, and 1 month after the index procedure. Before the index procedure, patients with AF had a significantly higher NYHA classification compared to patients with a SR. Regardless of the presence of AF, patient symptoms improved significantly after TAVI. However, the differences in NYHA classification between the AF and SR groups were maintained consistently after TAVI and at the 1-month follow-up (Fig. 1).
Fig. 1. Changes in symptoms before, after, and 1 month after TAVI according to AF. The interval plot shows the mean±95%CI of the NYHA class before, after, and 1 month after TAVI, according to AF. Small bar chart indicates the proportion of each NYHA class in the pre-TAVI, post-TAVI, and 1-month follow-up groups according to AF. SR group (blue) versus AF group (red) is shown. AF, atrial fibrillation; CI, confidence interval; NYHA, New York Heart Association; SR, sinus rhythm; TAVI, transcatheter aortic valve implantation.
Hemodynamic and echocardiography data
Before TAVI, patients with AF had a significantly lower LVEF compared to those with a SR. The severity of AS was similar between patients with and without AF. However, right ventricular systolic pressure, left atrium volume index, and proportion of moderate to severe mitral regurgitation were all significantly higher in patients with AF than in those with a SR (Table 2). At the immediate postoperative follow-up, the two groups maintained a similar trend of differences in their echocardiographic parameters. However, at the 1-month follow-up, only the left atrium volume index was significantly higher in the AF group than in the SR group, and the other hemodynamic and echocardiographic parameters were similar between patients with and without AF (Table 2).
Table 2. Baseline Hemodynamic and Echocardiographic Characteristics According to AF.
| Variables | SR (n=525) | AF (n=135) | p value | ||
|---|---|---|---|---|---|
| Pre-TAVI | |||||
| Systolic blood pressure, mm Hg | 128.0±19.7 | 126.7±21.9 | 0.500 | ||
| Diastolic blood pressure, mm Hg | 69.5±11.8 | 68.7±13.3 | 0.510 | ||
| Heart rate, /min | 72.6±14.4 | 74.6±16.0 | 0.159 | ||
| LVEDP, mm Hg | 20.6±11.3 | 21.4±8.2 | 0.523 | ||
| AV Vmax, m/s | 4.6±0.7 | 4.5±0.8 | 0.096 | ||
| AV mean pressure gradient, mm Hg | 52.9±17.0 | 50.4±18.2 | 0.120 | ||
| AV area, cm2 | 0.7±0.2 | 0.7±0.2 | 0.559 | ||
| AV area index, cm2/m2 | 0.5±0.1 | 0.5±0.3 | 0.446 | ||
| LVEF, % | 57.2±12.1 | 54.3±12.1 | 0.014 | ||
| E/e’ | 21.6±11.5 | 21.4±9.1 | 0.846 | ||
| RVSP, mm Hg | 37.6±12.0 | 43.3±13.8 | <0.001 | ||
| LAVI, mL/m2 | 52.9±18.9 | 71.4±42.5 | <0.001 | ||
| Moderate to severe mitral regurgitation | 50 (9.5) | 31 (23.0) | <0.001 | ||
| Moderate or severe aortic regurgitation | 74 (14.1) | 28 (20.7) | 0.076 | ||
| Post-TAVI | |||||
| Systolic blood pressure, mm Hg | 127.6±20.1 | 127.1±20.7 | 0.782 | ||
| Diastolic blood pressure, mm Hg | 63.3±12.3 | 64.6±13.4 | 0.304 | ||
| Heart rate, /min | 76.2±13.0 | 80.1±15.8 | 0.009 | ||
| LVEDP, mm Hg | 21.3±8.0 | 20.8±6.6 | 0.691 | ||
| AV Vmax, m/s | 2.4±0.8 | 2.3±0.5 | 0.222 | ||
| AV mean pressure gradient, mm Hg | 12.0±5.2 | 11.2±4.9 | 0.114 | ||
| AV EOA, cm2 | 1.8±0.5 | 1.8±0.5 | 0.268 | ||
| AV EOAI, cm2/m2 | 1.1±0.3 | 1.1±0.3 | 0.173 | ||
| LVEF, % | 59.1±10.0 | 57.0±10.2 | 0.033 | ||
| E/e’ | 21.5±9.9 | 21.2±9.7 | 0.779 | ||
| RVSP, mm Hg | 35.8±10.0 | 38.9±13.2 | 0.033 | ||
| LAVI, mL/m2 | 51.3±17.9 | 76.7±56.6 | <0.001 | ||
| Moderate or severe paravalvular leakage | 30 (5.7) | 9 (6.7) | 0.831 | ||
| Moderate or severe mitral regurgitation | 18 (3.4) | 16 (11.9) | <0.001 | ||
| 1-month follow-up | |||||
| Systolic blood pressure, mm Hg | 135.4±20.4 | 129.3±20.6 | 0.005 | ||
| Diastolic blood pressure, mm Hg | 68.9±12.3 | 68.4±12.8 | 0.719 | ||
| Heart rate, /min | 76.4±13.4 | 76.1±15.4 | 0.824 | ||
| AV Vmax, m/s | 2.3±0.5 | 2.2±0.5 | 0.068 | ||
| AV mean pressure gradient, mm Hg | 11.8±5.1 | 10.8±4.4 | 0.073 | ||
| AV EOA, cm2 | 1.7±0.5 | 1.7±0.5 | 0.931 | ||
| AV EOAI, cm2/m2 | 1.1±0.3 | 1.1±0.3 | 0.873 | ||
| LVEF, % | 60.5±8.9 | 59.4±9.0 | 0.274 | ||
| E/e’ | 20.5±9.5 | 20.4±9.0 | 0.985 | ||
| RVSP, mm Hg | 34.0±9.2 | 36.8±13.7 | 0.136 | ||
| LAVI, mL/m2 | 49.1±17.0 | 75.7±62.0 | 0.004 | ||
| Moderate to severe mitral regurgitation | 12 (2.3) | 7 (5.2) | 0.131 | ||
| Moderate or severe paravalvular leakage | 20 (3.8) | 2 (1.5) | 0.282 | ||
AF, atrial fibrillation; AV, aortic valve; EOA, effective orifice area; EOAI, effective orifice area index; LAVI, left atrium volume index; LVEDP, left ventricular end-diastolic pressure; LVEF, left ventricular ejection fraction; RVSP, right ventricular systolic pressure; SR, sinus rhythm; TAVI, transcatheter aortic valve implantation.
Data are presented as the mean±standard deviation or n (%).
Clinical outcomes according to AF
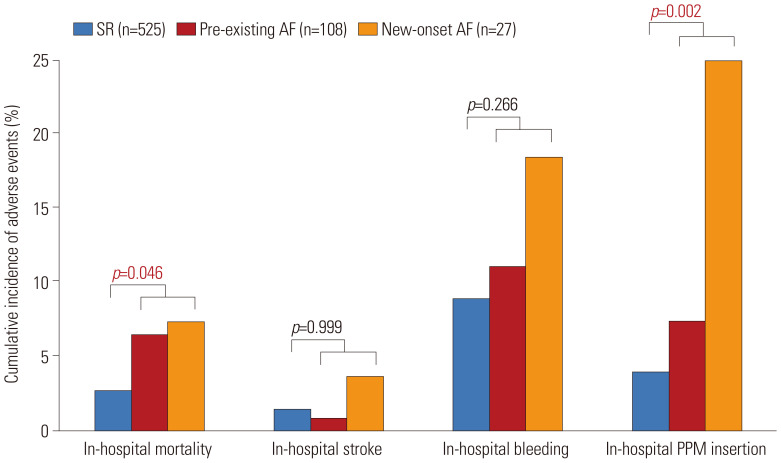
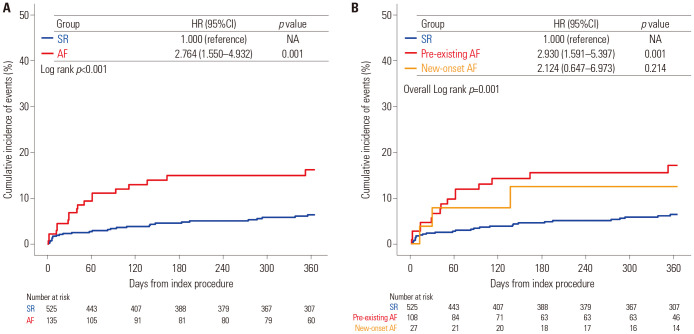
The median follow-up duration in the current registry was 369 days (interquartile range from 125 to 686 days). In-hospital mortality and the risk of pacemaker insertion were significantly higher in the AF group than in the SR group (Fig. 2). The presence of AF (pre-existing or new-onset) was associated with a significantly higher risk of 1-year all-cause mortality than was found in the SR group (16.2% vs. 6.4%, HR: 2.764, 95%CI: 1.550–4.932, p=0.001) (Table 3 and Fig. 3). The 1-year risks of cardiac death (9.1% vs. 1.9%, HR: 5.324, 95%CI: 2.100–13.500, p<0.001) and pacemaker insertion (14.0% vs. 5.5%, HR: 2.733, 95%CI: 1.483–5.039, p=0.001) were also significantly higher in patients with AF compared to those with a SR (Table 3 and Supplementary Fig. 2, only online). In subgroup analysis according to the type of AF, there was a significant increase of new pacemaker insertion in patients with new-onset AF (27.5% vs. 5.5%, HR: 6.329, 95%CI: 2.742–14.60, p<0.001) compared to those with SR, but not in patients with pre-existing AF (10.6% vs. 5.5%, HR: 1.960, 95%CI: 0.945–4.066, p=0.071) (Supplementary Table 3, only online). Pre-existing or new-onset AF, age, STS score, and NYHA classification before TAVI were independent predictors of all-cause mortality at 1 year (Table 4).
Fig. 2. In-hospital outcomes according to AF. Bar plot shows the proportion of clinical adverse events that occurred during the in-hospital period, according to AF. The SR group (blue) versus pre-existing AF group (red) versus new-onset AF group (orange) is shown. AF, atrial fibrillation; SR, sinus rhythm; PPM, permanent pacemaker.
Table 3. One-Year Clinical Outcomes According to AF.
| SR (n=525) | AF (n=135) | Univariate analysis | Multivariable analysis* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p value | HR | 95%CI | p value | ||||
| All-cause death | 29 (6.4) | 19 (16.2) | 2.764 | 1.550–4.932 | 0.001 | 2.207 | 1.182–4.120 | 0.013 | |
| Cardiac death | 8 (1.9) | 10 (9.1) | 5.324 | 2.100–13.500 | <0.001 | 4.442 | 1.572–12.556 | 0.005 | |
| Stroke | 11 (2.6) | 2 (1.7) | 0.776 | 0.172–3.502 | 0.741 | 0.429 | 0.084–2.200 | 0.310 | |
| Ischemic | 9 (2.1) | 1 (0.8) | 0.470 | 0.060–3.713 | 0.474 | 0.242 | 0.025–2.328 | 0.219 | |
| Hemorrhagic | 2 (0.5) | 1 (0.8) | 2.149 | 0.195–23.71 | 0.532 | 2.837 | 0.138–58.20 | 0.499 | |
| Bleeding | 52 (10.7) | 19 (15.4) | 1.471 | 0.869–2.487 | 0.150 | 1.399 | 0.798–2.543 | 0.242 | |
| Pacemaker insertion | 26 (5.5) | 17 (14.0) | 2.733 | 1.483–5.039 | 0.001 | 3.137 | 1.621–6.071 | 0.001 | |
AF, atrial fibrillation; AV, aortic valve; CI, confidence interval; HR, hazard ratio; LBBB, left bundle branch block; SR, sinus rhythm; STS, Society of Thoracic Surgery; Vmax, maximum velocity.
Values are n (%). The cumulative incidence of events is presented as Kaplan-Meier estimates.
*Adjusted variables included age, sex, hypertension, diabetes mellitus, previous history of stroke, previous myocardial infarction, previous cardiac surgery, LBBB, STS score, Euro-score II, CHAS2-VaSc score, combined coronary artery disease, AV Vmax, moderate or severe aortic regurgitation, moderate or severe mitral regurgitation, left ventricular ejection fraction, and New York Heart Association classification.
Fig. 3. Comparison of 1-year all-cause mortality according to AF. The Kaplan-Meier survival curves show the comparison of 1-year all-cause mortality between the SR group (blue) versus AF group (red) (A) and the SR group (blue) versus pre-existing AF group (red) versus new-onset AF group (orange) (B). AF, atrial fibrillation; HR, hazard ratio; CI, confidence interval; NA, not applicable; SR, sinus rhythm.
Table 4. Independent Predictors of All-Cause Mortality During Follow-Up.
| Multivariable analysis* | |||
|---|---|---|---|
| Adjusted HR | 95%CI | p value | |
| Atrial fibrillation | 2.207 | 1.182–4.120 | 0.013 |
| Age (per 5 increase) | 1.418 | 1.085–1.852 | 0.010 |
| STS score (per 1 increase) | 1.067 | 1.041–1.093 | <0.001 |
| NYHA Classification (per 1 increase) | 1.832 | 1.084–3.098 | 0.024 |
AV, aortic valve; CI, confidence interval; HR, hazard ratio; LBBB, left bundle branch block; NYHA, New York Heart Association; STS, The Society of Thoracic Surgery; Vmax, maximum velocity.
*Adjusted variables included age, sex, hypertension, diabetes mellitus, previous history of stroke, previous myocardial infarction, previous cardiac surgery, LBBB, STS score, Euro-score II, CHAS2-VaSc score, combined coronary artery disease, AV Vmax, moderate or severe aortic regurgitation, moderate or severe mitral regurgitation, left ventricular ejection fraction, and NYHA classification. C-index of Cox regression model was 0.813 (95%CI 0.746–0.880).
Predictors of new-onset AF after TAVI
To find the predictors of new-onset AF after TAVI, a multivariable logistic regression analysis was performed. Prolonged anesthesia, elevated creatinine before TAVI, and the CHAS2-VaSc score were independent predictors of new-onset AF after TAVI (Supplementary Table 4, only online).
Variation in antithrombotic regimen and clinical outcomes of AF patients after TAVI according to antithrombotic therapy
Of the 126 AF patients after TAVI, antithrombotic regimens were classified into three groups: 1) single-antithrombotic therapy for 28 (22.2%), which prescribed aspirin, clopidogrel, warfarin, or a NOAC; 2) dual-antithrombotic therapy for 77 (61.1%), which prescribed dual-antiplatelet therapy (aspirin and clopidogrel) or a combination of one antiplatelet agent (aspirin or clopidogrel) and one anticoagulant (warfarin or NOAC); and 3) triple-antithrombotic therapy for 21 (16.7%), which prescribed a combination of dual-antiplatelet therapy (aspirin and clopidogrel) and one anticoagulant (warfarin or NOAC). Over 50% of patients received anticoagulation therapy in both patients with history of percutaneous coronary intervention (PCI) (17/28, 60.7%) and those without a history of PCI (54/98, 55.1%). Among the patients without anticoagulation therapy, the most frequently prescribed combination was that of aspirin and clopidogrel (48/55, 87.3%), regardless of the history of PCI (Supplementary Table 5, only online).
The incidence of all-cause death and stroke within 1 year was numerically higher in the single-antithrombotic therapy group than in the dual and triple-antithrombotic therapy groups. On the other hand, follow-up bleeding within 1 year occurred more significantly in patients who were prescribed a triple-antithrombotic regimen than in those on a single or dual-antithrombotic regimen (Supplementary Fig. 3, only online).
DISCUSSION
This study used data from the nationwide K-TAVI registry to evaluate the impact of AF and antithrombotic management in Korean patients who underwent TAVI, and the principal findings were as follows. 1) Symptoms and echocardiographic parameters were consistently poorer in patients with AF, even after TAVI, compared to patients without AF. 2) Compared with a SR, AF was strongly associated with a higher risk of all-cause and cardiac mortality and new pacemaker insertion within 1 year, regardless of the onset timing of AF. 3) Among AF patients with AS who underwent TAVI, a substantial proportion of patients were received multiple antithrombotic agents and dual antiplatelet therapy with aspirin and clopidogrel was the most provided regimen.
Among patients with severe AS undergoing TAVI, the reported prevalence of AF varies from 16% to 50%.3,4,7,10 However, there has been a lack of data regarding the incidence of AF in Korean patients with severe AS undergoing TAVI. Here, we report that 16.4% of Korean patients with severe AS had pre-existing AF, and 4.1% of patients were diagnosed as new-onset AF after receiving TAVI. Compared with previous studies from Western countries, our cohort seems to be on the low end for the incidence of pre-existing and new-onset AF. The reason for such low prevalence of AF might be explained as follows. First, pre-existing AF is likely associated with the worse risk profile of TAVI candidates;7 therefore, a relatively low STS score or CHAS2-VaSc score in this study could reflect a lower incidence of pre-existing AF. Second, the transapical approach is a well-known risk factor for new-onset AF;8,10 therefore, the extremely low proportion of transapical approach procedures in our cohort might be related to the low incidence of new-onset AF. Third, the general prevalence of AF is known to be lower in Asia than in Europe and the United States.18
Recent studies investigating the prognostic implications of AF after TAVI have shown a consistent increase in early and late mortality in patients with pre-existing or new-onset AF.10,19,20 In accordance with those previous data, we observed a significantly higher risk of short-term (in-hospital) and 1-year follow-up mortality in AF patients after TAVI, regardless of the timing of AF onset. In addition to mortality, one of the important complications related to TAVI is an atrioventricular conduction disturbance that requires permanent pacemaker implantation. A previous meta-analysis showed that male sex, baseline conduction disturbances, and an intraprocedural atrioventricular block were predictors of pacemaker insertion after TAVI, but no increase in the risk of pacemaker implantation was identified for patients with AF.21 However, in a large retrospective cohort study of the STS/American College of Cardiology TVT Registry, baseline pre-existing AF was not associated with new pacemaker insertion for patients undergoing TAVI.22 Furthermore, data from the PARTNER trial demonstrated that the risk of new pacemaker insertion was significantly higher in patients with new-onset AF than in those with a SR or pre-existing AF.20 In agreement with the previous results, the present study also demonstrated that patients with AF experienced significantly more pacemaker insertion than those without AF, and this increased risk was attributable to patients with new-onset AF rather than pre-existing AF. Taken together, AF might be an independent predictor of new pacemaker insertion, mainly driven by new-onset AF; however, larger studies are required to examine the role of AF in pacemaker insertion among patients undergoing TAVI.
This study has several advantages and novel findings compared with previous studies. First, changes in dyspnea symptoms before, after, and 1 month after TAVI were systematically collected in this study. Interestingly, compared to patients with a SR, those with AF complained of more severe dyspnea symptoms before TAVI, despite having similar AS severity. Moreover, although the symptoms improved significantly after TAVI in all patients, regardless of the presence of AF, AF patients more commonly remained symptomatic. These results imply that AF itself contributes to dyspnea symptoms even in patients with severe AS, and thus, AF management is important even in patients who have undergone successful TAVI. Second, we comprehensively evaluated echocardiography data before, after, and 1 month after the index procedure. Although almost all previous studies provided baseline echocardiography parameters, follow-up echocardiography data are scarce. In the 1-month follow-up data of this study, we found no significant differences in echocardiographic parameters except for left atrium volume index, even though the LVEF, left atrium volume index, and right ventricular systolic pressure were significantly higher in AF patients than in non-AF patients before and after TAVI. Persistently higher left atrium volume index in AF patients, which is a well-known natural course of chronic AF, might be attributable to the larger proportion of pre-existing AF patients. Therefore, treating severe AS could be associated with improved LVEF and pulmonary hypertension even in patients with AF. To confirm our results, the collection of longer-term follow-up echocardiography data from a large sample size is warranted.
For patients with AS undergoing TAVI, cerebrovascular events are important factors that determine morbidity and mortality. Previous studies consistently demonstrated that cerebrovascular events were associated with an increased risk of early and late mortality in patients receiving TAVI.23,24 Nevertheless, the optimal antithrombotic regimen following TAVI is controversial. In particular, antithrombotic therapy for AF patients undergoing TAVI has not been established and lacks consensus. Although the latest guideline recommends lifelong OACs for TAVI patients who have other indications for OACs, such as AF,1,2 OACs are underused in more than 40% of patients with AF undergoing TAVI due to the difficulty of balancing the ischemic and bleeding risks.25 Likewise, in the present study, more than 40% of patients were prescribed only single or dual antiplatelet agents without OACs. Furthermore, in real-world practice, the management of antithrombotic therapy for AF patients after TAVI varies widely across centers and operators.15 Similarly, we found highly variable antithrombotic regimens among AF patients after TAVI. Although we found that a triple-antithrombotic regimen was associated with a significantly higher risk of bleeding without the benefit of ischemic events and patients with dual-antithrombotic regimen demonstrated numerically lower incidence of death and stroke within 1 year, our sample size was limited and underpowered to discuss the clinical outcomes according to antithrombotic therapy.
Several limitations of this study should be acknowledged. First, the non-randomized nature of the registry data could have produced selection bias. In particular, the choice of device, technique, and perioperative medications were all left to the individual operator’s discretion. The results according to antithrombotic therapy should be interpreted with caution due to confounding by operator’s discretion about antithrombotic agents. Second, although this study cohort is one of the largest collections of nationwide data focused on Korean patients, the number of cases was limited by the late arrival of TAVI in Korea. Due to the limited number of cases, the incidence of stroke was too small to evaluate the significant effect of stroke origin. Third, the pre-TAVI clinical severity of the candidates was relatively low compared with other cohorts.
In conclusion, AF in Korean patients undergoing TAVI correlated with significantly higher risks of short-term (in-hospital) mortality, 1-year follow-up all-cause mortality, and new pacemaker insertion compared to patients with a SR, regardless of the onset timing of AF. Nevertheless, the regimen of antithrombotic therapy was highly variable and lacked consensus. These results suggest that AF is a prognostic parameter for TAVI and that detailed management with optimal antithrombotic therapy is necessary for AF patients undergoing TAVI.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Seung-Hyuk Choi and Hyeon-Cheol Gwon.
- Data curation: Sang Yoon Lee and Ki Hong Choi.
- Formal analysis: Sang Yoon Lee and Ki Hong Choi.
- Investigation: Sang Yoon Lee and Ki Hong Choi.
- Methodology: Ki Hong Choi and Seung-Hyuk Choi.
- Project administration: Seung-Hyuk Choi.
- Resources: Ki Hong Choi, Take Kyu Park, Jihoon Kim, Eun Kyoung Kim, Sung-Ji Park, Seung Woo Park, Hyeon-Cheol Gwon, Kiyuk Chang, Cheol Woong Yu, JuHan Kim, Young Jin Choi, In-Ho Chae, Jae-Hwan Lee, Jun-Hong Kim, Jong Seon Park, Won-Jang Kim, Young Won Yoon, Tae Hoon Ahn, Sang Rok Lee, Byoung Joo Choi, Tae-Hyun Yang, Cheol Ung Choi, Seung-Ho Hur, Seong-Jin Oh, Han Cheol Lee, HunSik Park, Hyo-Soo Kim, and Seung-Hyuk Choi.
- Software: Sang Yoon Lee and Ki Hong Choi.
- Supervision: Ki Hong Choi and Seung-Hyuk Choi.
- Validation: Sang Yoon Lee and Ki Hong Choi.
- Visualization: Sang Yoon Lee and Ki Hong Choi.
- Writing—original draft: Sang Yoon Lee, Ki Hong Choi, and Seung-Hyuk Choi.
- Writing—review & editing: Take Kyu Park, Jihoon Kim, Eun Kyoung Kim, Sung-Ji Park, Seung Woo Park, Hyeon-Cheol Gwon, Kiyuk Chang, Cheol Woong Yu, JuHan Kim, Young Jin Choi, In-Ho Chae, Jae-Hwan Lee, Jun-Hong Kim, Jong Seon Park, Won-Jang Kim, Young Won Yoon, Tae Hoon Ahn, Sang Rok Lee, Byoung Joo Choi, Tae-Hyun Yang, Cheol Ung Choi, Seung-Ho Hur, Seong-Jin Oh, Han Cheol Lee, HunSik Park, and Hyo-Soo Kim.
- Approval of final manuscript: all authors.
SUPPLEMENTARY MATERIALS
Baseline Clinical and Procedural Characteristics According to Onset Timing of AF
Baseline Hemodynamic and Echocardiographic Characteristics According to Onset Timing of AF
One-Year Clinical Outcomes According to AF Onset Timing
Independent Predictors of New-Onset AF after TAVI
Specific Type of Antithrombotic Therapy in Patients with AF after TAVI
Study flow. A flow diagram is shown. AF, atrial fibrillation; AS, aortic stenosis; PPM, permanent pacemaker; TAVI, transcatheter aortic valve implantation.
Comparison of 1-year clinical outcomes according to AF. Kaplan-Meier curves are shown for comparison of risks of cardiac death (A), stroke (B), new PPM insertion (C), and bleeding (D) in the study population. AF, atrial fibrillation; PPM, permanent pacemaker; SR, sinus rhythm.
Comparison of 1-year cumulative incidence of adverse events according to antithrombotic therapy after discharge in AF patients undergoing TAVI. Bar plot shows the cumulative incidence of adverse events according to the antithrombotic therapy regimen after discharge of AF patients undergoing TAVI. Single-antithrombotic therapy (blue), dual-antithrombotic therapy (red), and triple-antithrombotic therapy (orange) groups are shown. AF, atrial fibrillation; TAVI, transcatheter aortic valve implantation.
References
- 1.Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021;60:727–800. doi: 10.1093/ejcts/ezab389. [DOI] [PubMed] [Google Scholar]
- 2.Writing Committee Members. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP, 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. J Am Coll Cardiol. 2021;77:e25–e197. doi: 10.1016/j.jacc.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 3.Mentias A, Saad M, Girotra S, Desai M, Elbadawi A, Briasoulis A, et al. Impact of pre-existing and new-onset atrial fibrillation on outcomes after transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2019;12:2119–2129. doi: 10.1016/j.jcin.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sannino A, Gargiulo G, Schiattarella GG, Perrino C, Stabile E, Losi MA, et al. A meta-analysis of the impact of pre-existing and new-onset atrial fibrillation on clinical outcomes in patients undergoing transcatheter aortic valve implantation. EuroIntervention. 2016;12:e1047–e1056. doi: 10.4244/EIJY15M11_12. [DOI] [PubMed] [Google Scholar]
- 5.Filardo G, Hamilton C, Hamman B, Hebeler RF, Jr, Adams J, Grayburn P. New-onset postoperative atrial fibrillation and long-term survival after aortic valve replacement surgery. Ann Thorac Surg. 2010;90:474–479. doi: 10.1016/j.athoracsur.2010.02.081. [DOI] [PubMed] [Google Scholar]
- 6.Jørgensen TH, Thygesen JB, Thyregod HG, Svendsen JH, Søndergaard L. New-onset atrial fibrillation after surgical aortic valve replacement and transcatheter aortic valve implantation: a concise review. J Invasive Cardiol. 2015;27:41–47. [PubMed] [Google Scholar]
- 7.Tarantini G, Mojoli M, Urena M, Vahanian A. Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: epidemiology, timing, predictors, and outcome. Eur Heart J. 2017;38:1285–1293. doi: 10.1093/eurheartj/ehw456. [DOI] [PubMed] [Google Scholar]
- 8.Amat-Santos IJ, Rodés-Cabau J, Urena M, DeLarochellière R, Doyle D, Bagur R, et al. Incidence, predictive factors, and prognostic value of new-onset atrial fibrillation following transcatheter aortic valve implantation. J Am Coll Cardiol. 2012;59:178–188. doi: 10.1016/j.jacc.2011.09.061. [DOI] [PubMed] [Google Scholar]
- 9.Chopard R, Teiger E, Meneveau N, Chocron S, Gilard M, Laskar M, et al. Baseline characteristics and prognostic implications of pre-existing and new-onset atrial fibrillation after transcatheter aortic valve implantation: results from the FRANCE-2 registry. JACC Cardiovasc Interv. 2015;8:1346–1355. doi: 10.1016/j.jcin.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Tarantini G, Mojoli M, Windecker S, Wendler O, Lefèvre T, Saia F, et al. Prevalence and impact of atrial fibrillation in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement: an analysis from the SOURCE XT prospective multicenter registry. JACC Cardiovasc Interv. 2016;9:937–946. doi: 10.1016/j.jcin.2016.01.037. [DOI] [PubMed] [Google Scholar]
- 11.Stortecky S, Buellesfeld L, Wenaweser P, Heg D, Pilgrim T, Khattab AA, et al. Atrial fibrillation and aortic stenosis: impact on clinical outcomes among patients undergoing transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2013;6:77–84. doi: 10.1161/CIRCINTERVENTIONS.112.000124. [DOI] [PubMed] [Google Scholar]
- 12.Shahim B, Malaisrie SC, George I, Thourani VH, Biviano AB, Russo M, et al. Postoperative atrial fibrillation or flutter following transcatheter or surgical aortic valve replacement: PARTNER 3 trial. JACC Cardiovasc Interv. 2021;14:1565–1574. doi: 10.1016/j.jcin.2021.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Mojoli M, Gersh BJ, Barioli A, Masiero G, Tellaroli P, D’Amico G, et al. Impact of atrial fibrillation on outcomes of patients treated by transcatheter aortic valve implantation: a systematic review and meta-analysis. Am Heart J. 2017;192:64–75. doi: 10.1016/j.ahj.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP, 3rd, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. J Am Coll Cardiol. 2017;70:252–289. doi: 10.1016/j.jacc.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 15.Rossi JE, Noll A, Bergmark B, McCabe JM, Nemer D, Okada DR, et al. Variability in antithrombotic therapy regimens peri-TAVR: a single academic center experience. Cardiol Ther. 2015;4:197–201. doi: 10.1007/s40119-015-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Eur Heart J. 2010;31:2369–2429. doi: 10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 17.Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the valve academic research consortium-2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. doi: 10.1016/j.jacc.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 18.Rodriguez CJ, Soliman EZ, Alonso A, Swett K, Okin PM, Goff DC, Jr, et al. Atrial fibrillation incidence and risk factors in relation to race-ethnicity and the population attributable fraction of atrial fibrillation risk factors: the multi-ethnic study of atherosclerosis. Ann Epidemiol. 2015;25:71–76.e1. doi: 10.1016/j.annepidem.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chopard R, Teiger E, Meneveau N, Chocron S, Gilard M, Laskar M, et al. Baseline characteristics and prognostic implications of pre-existing and new-onset atrial fibrillation after transcatheter aortic valve implantation: results from the FRANCE-2 registry. JACC Cardiovasc Interv. 2015;8:1346–1355. doi: 10.1016/j.jcin.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Biviano AB, Nazif T, Dizon J, Garan H, Fleitman J, Hassan D, et al. Atrial fibrillation is associated with increased mortality in patient undergoing transcatheter aortic valve replacement: insights from the placement of aortic transcatheter valve (PARTNER) trial. Circ Cardiovasc Interv. 2016;9:e002766. doi: 10.1161/CIRCINTERVENTIONS.115.002766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siontis GC, Jüni P, Pilgrim T, Stortecky S, Büllesfeld L, Meier B, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J Am Coll Cardiol. 2014;64:129–140. doi: 10.1016/j.jacc.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 22.Fadahunsi OO, Olowoyeye A, Ukaigwe A, Li Z, Vora AN, Vemulapalli S, et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacement: analysis from the U.S. Society of Thoracic Surgeons/American College of Cardiology TVT registry. JACC Cardiovasc Interv. 2016;9:2189–2199. doi: 10.1016/j.jcin.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Kapadia S, Agarwal S, Miller DC, Webb JG, Mack M, Ellis S, et al. Insights into timing, risk factors, and outcomes of stroke and transient ischemic attack after transcatheter aortic valve replacement in the PARTNER trial (placement of aortic transcatheter valves) Circ Cardiovasc Interv. 2016;9:e002981. doi: 10.1161/CIRCINTERVENTIONS.115.002981. [DOI] [PubMed] [Google Scholar]
- 24.Nombela-Franco L, Webb JG, de Jaegere PP, Toggweiler S, Nuis RJ, Dager AE, et al. Timing, predictive factors, and prognostic value of cerebrovascular events in a large cohort of patients undergoing transcatheter aortic valve implantation. Circulation. 2012;126:3041–3053. doi: 10.1161/CIRCULATIONAHA.112.110981. [DOI] [PubMed] [Google Scholar]
- 25.Sherwood MW, Gupta A, Vemulapalli S, Li Z, Piccini J, Harrison JK, et al. Variation in antithrombotic therapy and clinical outcomes in patients with preexisting atrial fibrillation undergoing transcatheter aortic valve replacement: insights from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circ Cardiovasc Interv. 2021;14:e009963. doi: 10.1161/CIRCINTERVENTIONS.120.009963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Baseline Clinical and Procedural Characteristics According to Onset Timing of AF
Baseline Hemodynamic and Echocardiographic Characteristics According to Onset Timing of AF
One-Year Clinical Outcomes According to AF Onset Timing
Independent Predictors of New-Onset AF after TAVI
Specific Type of Antithrombotic Therapy in Patients with AF after TAVI
Study flow. A flow diagram is shown. AF, atrial fibrillation; AS, aortic stenosis; PPM, permanent pacemaker; TAVI, transcatheter aortic valve implantation.
Comparison of 1-year clinical outcomes according to AF. Kaplan-Meier curves are shown for comparison of risks of cardiac death (A), stroke (B), new PPM insertion (C), and bleeding (D) in the study population. AF, atrial fibrillation; PPM, permanent pacemaker; SR, sinus rhythm.
Comparison of 1-year cumulative incidence of adverse events according to antithrombotic therapy after discharge in AF patients undergoing TAVI. Bar plot shows the cumulative incidence of adverse events according to the antithrombotic therapy regimen after discharge of AF patients undergoing TAVI. Single-antithrombotic therapy (blue), dual-antithrombotic therapy (red), and triple-antithrombotic therapy (orange) groups are shown. AF, atrial fibrillation; TAVI, transcatheter aortic valve implantation.