Abstract
Background
Wedelolactone, main active constituent of Wedelia calendulace and Eclipta alba plants which has been traditionally used to treat various chronic inflammatory conditions. However, its mechanism of action of anti-inflammatory effect on ulcerative colitis is yet to be established.
Objective
In the present study, the effect of the wedelolactone on the myeloperoxidase activities and in the production of proinflammatory cytokines involved in the pathogenesis of chronic inflammation was assessed.
Materials and methods
Wistar rats were randomly divided into four groups containing six animals per group. Group I (Vehicle control): tap water and vehicle; Group II (DSS control): tap water containing 5% (w/v) of DSS over 7 days, and vehicle; Group III (treatment group): Wedelolactone 50 mg/kg/day, and tap water containing 5% DSS over 7 days, Group IV (treatment group): Wedelolactone 100 mg/kg/day and tap water containing 5% DSS over 7 days over the experiment.
Results
Study revealed that wedelolactone treatment dramatically decrease the release of IL-1a, IL-1b, IL-2, TNF, INFγ, STAT3 and CCL-5 in colons treated with DSS. In summary, these results suggest that the inhibition of IL-6/STAT3 signaling is a potential mechanism by which wedelolactone is used in the treatment of ulcerative colitis.
Conclusion
Oral administration of Wedelolactone (100 mg/kg) significantly attenuated pathological colonic damage and inhibited inflammatory infiltration, myeloperoxidase activities. In summary, Wedelolactone showed anti-inflammatory effect by down regulation of the IL-6/STAT3 inflammatory signaling pathway. These findings provide new insights into the pharmacological actions of wedelolactone as a potential therapeutic agent for colitis.
Keywords: Wedelolactone, Dextran sulphate sodium, Colitis, Pro-inflammatory cytokines, IL-6/STAT3 pathway
1. Introduction
Wedelolactone (WEL) is a plant-derived coumarin isolated from Wedelia chinensis [Vernacular name: Pitabhrang, Pilabhangra, Bhangra, Bhanra (Hindi); Gargari (Kannada); Pitabhrangaraja, Pitabhranga (Sanskrit)]family Asteraceae (Compositae), it has many biological effects including anti-tumor, anti-osteoporosis, antifibrotic, anti-HCV and cholesterol-reducing activities [1]. Ulcerative colitis (UC) and Crohn's disease are two conditions known collectively as inflammatory bowel disease. The incidence of UC is estimated to vary from 0.5 to 24.5 per 100,000 inhabitants worldwide [2]. It is well recognized that UC is a chronic inflammatory disease with unknown etiology and partially understood pathogenesis. It is recognized as important cause of gastrointestinal disease in children and adults. Ulcerative colitis is a colon affected idiopathic disease characterized by ulceration of the colon mucosa causing bloody diarrhoea, abdominal pain and rectal bleeding [3]. UC is the most common type of inflammatory disease of the intestine worldwide [4]. Ulcerative colitis (UC) and Crohn disease (CD) are major forms of the non-specific chronic inflammatory bowel disease (IBD) with unknown etiology. Studies suggest that main disease segments of UC are the colon/rectum while those of CD are terminal ileum or colon [5]. Usually, CD affects any part of the gastrointestinal tract with characteristic skip lesions. On the other hand, UC affects only the rectum and the colon, with the lesions being more homogeneous and continuous [6]. The choice of treatment depends on severity, localization and the course of the disease. For proctitis, topical therapy with 5-aminosalicylic acid (5-ASA: Sulfasalazine, Mesalamine, Olsalazine, Balsalazide) compounds is used. More extensive or severe disease should be treated with oral and local 5-ASA compounds and corticosteroids to induce remission. Patients who do not respond to this treatment require hospitalization. Intravenous steroids or, when refractory, calcineurin inhibitors (cyclosporine, tacrolimus), tumor necrosis factor-α antibodies (infliximab) or immunomodulators (Azathioprine, 6-mercaptopurine, Cyclosporine, Tacrolimus), Boilogic and biosimilars therapies (Adalimumab, Golimumab, Infliximab, Ustekinumab, Vedolizumab, Infliximab-abda, Infliximab-dyyb) in combination with rehydration and symptomatic treatment using antidiarrheal agents.
Dextran Sulphate Sodium (DSS) induced colitis model is one of the commonly used models because it can be easily developed for the wide accessibility and low price of DSS. DSS administration is a popular technique for the growth of ulcer and inflammation in animals. DSS primarily affects the large intestine either centre and distal third of the intestine. It also affects the distal small intestine i.e ileum [7]. The object of present study was to explore the effects of wedelolactone on inflammation of the intestines (see Fig. 1).
Fig. 1.
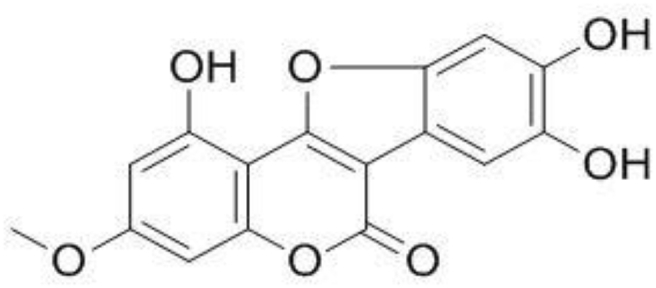
Chemical Structure of Wedelolactone.
2. Materials and methods
2.1. Wedelolactone (drug profile)
Wedelolactone (MW: 314.25 g/mol & Synonym: 7-Methoxy-5,11,12-trihydroxycoumestan1,8,9-Trihydroxy-3-methoxycoumestan). The main source is Eclipta alba (false daisy), [8] Wedeliacalendulacea [9] and Wedelia chinensis. It is sparingly soluble in aqueous buffers.
2.2. Drug, chemicals, ELISA kits
Wedelolactone (purity > 98%) was prepared in Baoji Guokang Bio-Technology CO., LTD, China, DSS (molecular weight 36,000–50,000 Da, CAT NO: 160,110) was purchased from Sigma Aldrich (USA). ELISA Kit was purchased from Lab Pro, Bangalore & Krishgen BioSystems, Mumbai. Other chemical products used were of the analytical grade available.
2.3. Animals
Female Albino Wistar rats were procured from Biogen Laboratory Animal Facility, Bangalore, weighing 150–180 g. The animals were allowed access to food and tap water ad libitum throughout the acclimatization and experimental periods. All animal experiments were carried out in accordance with the CPCSEA guide for the care and use of laboratory animals. This study was approved by the Institutional Animal Ethics Committee (Ref: IAEC/ABMRCP/2016–17/PR/09) of the Acharya & BM Reddy College of Pharmacy, Bangalore.
2.4. DSS-induced acute colitis in rats
Acute colitis in rats was induced by adding DSS to the drinking water at a concentration of 5% (w/v) and administered to group II to group IV for a period of 7 days. Test rats were treated orally with either vehicle solution or Wedelolactone from the beginning of the experiment for first 3 days, followed by provision of 5% DSS in drinking water up to 7 days. The rats were divided into 4 groups: Group I- Vehicle control: Received only the vehicle (0.1 ml/10 g/day, oral) on day 1 to day 3 and allowed to drink tap water over 7 days. Group II - DSS control: Received only the vehicle (0.1 ml/10 g/day, oral) on day 1 to day 3 and allowed to drink tap water containing 5% (w/v) of DSS over 7 days. Group III - Treatment group: Wedelolactone administered at dose of 50 mg/kg/day, single dose, orally on day 1 to day 3 and allowed to drink tap water containing 5% DSS over 7 days, Group IV - Treatment group: Wedelolactone administered at dose of 100 mg/kg/day, single dose, orally on day 1 to day 3 and allowed to drink tap water containing 5% DSS over 7 days over the experiment. Rats in the DSS group soon developed typical symptoms of clinical colitis, including diarrhoea, rectal bleeding and loss of body weight starting from day 3. Animal body weights, stool consistency and the presence of gross blood in feces were recorded daily throughout all the experiments. Animals were sacrificed on day 8 by cervical dislocation. Rat abdomen were dissected and entire portions of colon were dissected out, washed with normal saline several time and measured the colon length with digital calibre. Parts of colon tissues were stored at 80 °C for biochemical analysis. Portions of colons were also fixed in 10% (v/v) neutral buffered formalin solutions for 24 h for histopathological analysis.
2.5. Clinical scoring
DSS-induced colitis was scored with the Disease Activity Index (DAI). The DAI score was obtained based on weight loss, stool consistency change, and bleeding in stool. Briefly, the following parameters were used for calculation: a) body weight loss (0 point = no loss, 1 point = 1–5% loss, 2 points = 6–10% loss, 3 points = 10–20% loss, 4 points = over 20% loss); b) diarrhea (0 point = normal, 2 points = loose stools, 4 points = watery diarrhea); c) hematochezia (0 point = no bleeding, 2 points = slight bleeding, 4 points = gross bleeding). Standard histopathological techniques were followed for processing portions of distal colon, preparation of paraffin blocks and staining of the slides with hematoxylin–eosin to evaluate DSS induced colitis.
2.6. MPO determination
Tissue MPO activity, which are linearly related to inflammatory infiltration in inflamed tissue, were assayed to monitor the degree of inflammation. MPO activity was measured in pieces of colon. MPO assay was used to indirectly assess the infiltration of neutrophils to the swollen colon the assay findings were expressed in ng/ml. MPO activity was determined by the modified method. The colon tissues were homogenized in 0.5% hexadecyltrimethylammonium bromide 0.5 mL/50 mg of colon tissue; then the homogenates were centrifuged at 18,000 g for 15 min at 4 °C. Aliquots of 40 ml supernatant were mixed with 60 μL potassium phosphate buffer (50 mmol, pH 6.0) with o-dianisidinedihydrochloride and hydrogen peroxide. MPO activity was found from the rate of absorbance alteration in 3 min at 460 nm and calculation was done using the following formula [10].
A - Difference in absorbance, Vt - Total volume, E − Extinction coefficient, Δt − Measuring time, VS − Sample volume.
2.7. Analysis of cytokine profiles in colons
At the end of the experiment, the colon was removed and washed with phosphate-buffered saline (PBS). Distal colons were homogenized in ice-cold PBS (containing 0.05% Tween 20, 0.1 mM phenylmethylsulfonyl fluoride, 0.1 mM benzethonium chloride and 10 mM EDTA). The homogenate was centrifuged (3000 g) for 10 min, and the supernatants were stored at 80 °C. The levels of cytokines IL-1α, IL-1β, IL-2, IL-6, TNF-α, STAT3, IFNγ, NFκB and CCL-5 were determined using the ELISA kits as described by the manufacturer.
2.8. Histopathological assessment of colitis severity
The colon tissues were fixed in 10% phosphate-buffered formaline, regularly processed for paraffin embedding, sliced at 5 μm and stained with haematoxyline. The histopathological study was conducted with the consideration of parameters such as tissue loss/cell death, eosin for colonic architecture assessment, severity of injury and mucosal epithelial lesion, infiltration of lymphocytes, inflammation and mucosal injury. Longitudinal sections of distal colon were assessed.
2.9. Statistical analysis
Data were expressed as means ± SEM from at least three independent experiments. Statistical significance between different groups was analyzed by one-way analysis of variance (ANOVA) followed by Dunnett's test between control group and multiple dose groups. Values of p < 0.05 were regarded statistically significant.
3. Results
3.1. Effect of wedelolactone on body weight
Effects of wedelolactone on body weight in rats with colitis is presented in Table 1. The body weight in DSS induced colitis rats was decreased about 7.84% and 10.87% at 3 day and 7 day, respectively, when compared to that of the respective group on day 0, The body weight was recovered on 7th day at both 50 and 100 mg/kg body wedelolactone treated group (1.31% & 1.89%) but not statistically significant.
Table 1.
Effect of Wedelolactone on body weight.
| Groups | Treatment | Changes in body weight Mean ± SEM |
||
|---|---|---|---|---|
| Day 0 | Day 3 | Day 7 | ||
| I | Vehicle control | 173.33 ± 6.14 | 173.33 ± 4.70 | 176.66 ± 4.04 |
| II | DSS colitis control | 170.00 ± 1.66 | 156.66 ± 3.07 (7.84↓) | 153.33 ± 4.04 (10.87↓) |
| III | Wedelolactone treatment group (50 mg/kg) | 156.41 ± 1.16 | 154.33 ± 4.56 (1.32 ) | 154.36 ± 3.87 (1.31 ) |
| IV | Wedelolactone treatment group (100 mg/kg) | 158.33 ± 3.33 | 140.00 ± 2.11 (11.57 ) | 155.33 ± 2.58 (1.89 ) |
The values are expressed as Mean ± SEM (n = 6). ∗∗p < 0.01, ∗∗∗p < 0.001, Data was analysed by One-Way Analysis of Variance (ANOVA) followed by Dunnett's test. In parenthesis it showed % of decreased (↓)/increased (↑) in body weight.
3.2. Effect of wedelolactone on DAI and colitis score
The DAI score is a common parameter used for evaluating severity of colitis. The higher DAI score suggests the severe state of colitis. Compared with the normal control group, there was significant increase in DAI score of DSS group indicating that the DSS group showed substantial loss of body weight, diarrhea and bloody stool. Consistent with the findings from changes in body weight, the results from stool consistency score indicated that colitis animals had raised score compared to the controls. Administration of wedelolactone markedly decreased the stool consistency score. Statistically significant reduction was observed in 50 and 100 mg/kg groups compared to the control group (Table 2). The colitis scoring was recorded on day 15 and data is presented in Table 2, it was significantly increased in DSS induced colitis control group (colitis score was 9.33 out of 10 on day 15) compared to vehicle control animals. The colitis score of wedelolactone (50 & 100 mg/kg) treated animals was 5.02 & 2.00 out of 10 on day 15 and significantly (p < 0.001) decreased compared with DSS colitis control animals.
Table 2.
Effect of Wedelolactone on Disease activity index (DAI) and Colitis Score of DSS induced colitis.
| Groups | Treatment | DAI (Day 3) | DAI (Day 7) | Colitis Score |
|---|---|---|---|---|
| I | Vehicle control | 00.00 ± 00.00 | 00.00 ± 00.00 | 00.00 ± 00.00 |
| II | DSS control | 3.640 ± 0.81 | 2.440 ± 0.50 | 9.33 ± 0.25 |
| III | Wedelolactone treatment group (50 mg/kg) | 2.308 ± 0.26 ∗∗ | 1.220 ± 0.33 | 5.02 ± 0.31 ∗∗∗ |
| IV | Wedelolactone treatment group (100 mg/kg) | 2.10 ± 0.36 ∗∗ | 0.775 ± 0.24 ∗∗ | 2.00 ± 0.22 ∗∗∗ |
The values are expressed as Mean ± SEM (n = 6). ∗∗p < 0.01, ∗∗∗p < 0.001, Data was analysed by One-Way Analysis of Variance (ANOVA) followed by Dunnett's test.
3.3. Effect of Wedelolactone on Myeloperoxidase
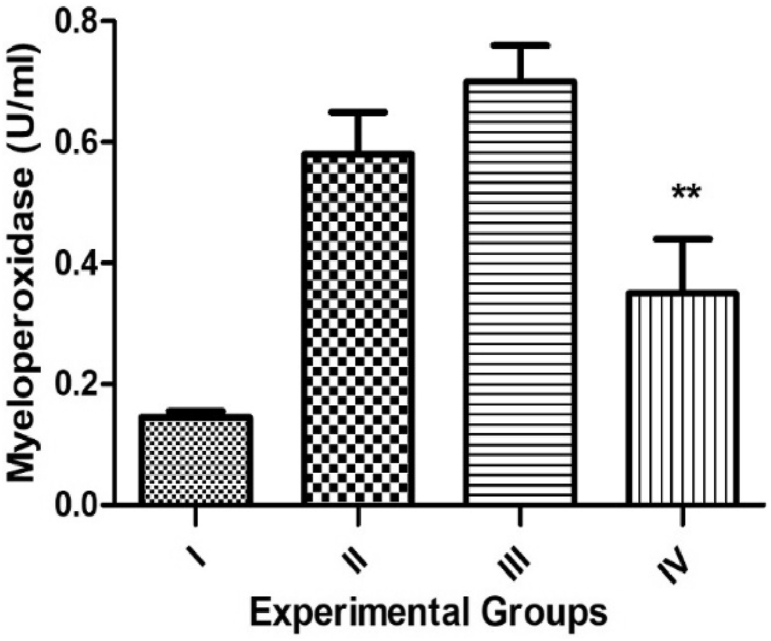
The colonic MPO activity also denotes index of inflammation. Myeloperoxidase enzymes were assessed in all experimental groups as a sign of neutrophil influx. DSS administration resulted in increased MPO activity, indicative of neutrophil infiltration and significantly increase (p < 0.001) in colitis rats (Fig. 2). However, wedelolactone treated rats presented a decrease in MPO concentration at 100 mg/kg dose (p < 0.01).
Fig. 2.
Effect of Wedelolactone on Myeloperoxidase assay (MPO) of DSS induced. Group I-Vehicle group, Group II-DSS control, Group III-Wedelolactone treatment group (50 mg/kg), Group IV-Wedelolactone treatment group (100 mg/kg). The values are expressed as Mean ± SEM (n = 6), ∗∗p < 0.01, Data was analysed by One-Way Analysis of Variance (ANOVA) followed by Dunnett's test.
3.4. Effect of wedelolactone on cytokine production in colon
After experimentally colitis induction by DSS, deregulated cytokines in pathological conditions promoting a pro-inflammatory effect, such as IL-1α, IL-1β, IL-2, IL-6, TNF-α, STAT3, IFNγ, NFκB and CCL-5. Rats with DSS induced colitis revealed a significant increase in IL-1α, IL-1β, IL-2, IL-6, TNF-α, STAT3, IFNγ, NFκB and CCL-5 compared to the vehicle control rats (p < 0.001) indicates a systemic diseased condition (Fig. 3a–3i). However, wedelolactone treated (50 & 100 mg/kg) rats showed a significant decrease in pro-inflammatory cytokines compared with the DSS colitis group (p < 0.01 & p < 0.001). These results indicate that wedelolactone (50 &100 mg/kg) prevent inflammatory reaction through down regulating cytokines expression and data are presented in Fig. 3(a–i).
Fig. 3.
Effect of Wedelolactone on Pro-inflammatory cytokines [(a) IL-1α, (b) IL-1β, (c) IL-2, (d) IL-6, (e) TNF-α, (f) IFNγ, (g) STAT3, (h) CCL-5 and (i) NFκB)] of DSS induced colitis. Group I-Vehicle control, Group II-DSS control, Group III-Wedelolactone treatment group (50 mg/kg), Group IV-Wedelolactone treatment group (100 mg/kg). The values are expressed as Mean ± SEM (n = 6). ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗p < 0.001, Data was analyzed by One-Way Analysis of Variance (ANOVA) followed by Dunnett's test.
3.5. Histopathological assessment of colitis severity
Representative images for the study groups interpreting the histopathological scores are presented in Fig. 4. The DSS exhibited significant haemorrhaging of diffuse transmural necrosis, involving mucosa, submucosa, muscle layer and serosa, and linked with peritonitis (Fig. 4b). Similar lesions were observed in the rats treated with wedelolactone, namely transmural necrosis but to a slighter degree and with a multifocal form, scattered with areas where the integrity of the mucosa was preserved (Fig. 4c). There was mild to moderate epithelial erosion and ulceration in these areas, and severe inflammatory cell infiltration was observed which extended to the submucosa (Fig. 4d). Normal group (Fig. 4a) not exhibited epithelial erosion, inflammatory cell infiltration and lesions, epithelial erosion and ulceration.
Fig. 4.
Histopathological changes in the experimental groups. Each image corresponding with a different experimental group, namely; (a) Group I-Vehicle control, (b) Group II-DSS control, (c) Group III-Wedelolactone treatment group (50 mg/kg), (d) Group IV-Wedelolactone treatment group (100 mg/kg).
4. Discussion
DSS does not directly cause inflammation of the intestine, it causes chemical damage to the intestinal epithelium, leading to exposure of the lamina propria (LP) and submucosal compartment to luminal antigen and enteric bacteria, leading to inflammation [11].
DSS leads to acute colitis that is morphologically and macroscopically described by hyperaemia, ulceration, moderate to serve sub mucosal edema, lesions accompanied by histopathological modification including granulocyte infiltration, symptoms of which are eventually expressed as bloody diarrhoea [12]. It considerably increases the output of all proinflammatory cytokines in both the middle and distal colon, but DSS induced UC in the distal colon appears to be more serious [13].
Myeloperoxidase (MPO) serves as a good marker of inflammation, tissue injury and neutrophil infiltration into gastrointestinal tissues. This model can also be used to study inflammatory cells that infiltrate the intestine, accumulation of neutrophil is the inflamed intestinal mucosa is a prominent feature in ulcerative colitis. The mechanisms involved in chemotaxis and transmigration of inflammatory cells and the various pathways involved in the activation and release of other proinflammatory mediators involved in tissue injury as well as mediators involved in modulating the inflammatory response and in tissue repair [14]. Wedelolactone significantly decreases the MPO concentration in colitis rats, so that it can corelate wedelolactone reduce neutrophil infiltration in the colitis tissue.
Pro-inflammatory cytokine IL-1b plays an important role in the pathogenesis of UC; high levels of IL-1b secreted by macrophages are correlated with the severity of inflammation in colitis [15], its production relies on inflammation activation and high plasma level of IL-1b correlates well with the severity of intestinal inflammation and disease activity [16]. IL-1b dramatically increased in the serum and inflamed colonic tissues of IBD patients. In our animal model of DSS induced colitis, mature IL-1b production was also significantly upregulated. Excessive IL-1b was reported to cause the increase in intestinal permeability [17].
IL-1a was the key Intestinal epithelial cell (IEC) derived necrotic cell product involved in human intestinal fibroblasts (HIF) cytokine production. IL-1a may be a target for treating early IBD or preventing the reactivation of IBD [18].
NF-κB activation can link inflammation to cancer, by regulating the functions of inflammatory cells. NF-κB activation can induce TNF expression and TNF is a potent activator of NF-κB. The elevation in TNF-alpha level is the hallmark of DSS induced colitis. It causes disturbance in the metabolism of glycerophosphocholine in the colon of the mice [19]. TNF protein was detected mainly in mononuclear cells present in the lamina propria and submucosal region. Inflammatory chemokines are released by a wide range of cell types in response to pro-inflammatory cytokines, tissue injury, or contact with pathogens [20]. NF-κB has a protective role in intestinal epithelial cells, where it is required for maintaining epithelial integrity and intestinal immune homeostasis. TNF was similarly observed in the colons of patients with active ulcerative colitis and advanced colorectal cancer [21].
NF-kB and MAPKs are signal messengers that regulate pro-inflammatory gene transcription and adhesion molecules in the inflammatory process [22]. Dysregulation of epithelial barrier function can lead to increased intestinal permeability, which can contribute to the release of systemic inflammatory mediators, leading to the development of inflammation. The metabolic regulator AMP-activated protein kinase (AMPK) has been reported to play a role in the regulation of intestinal barrier function and inflammation [23]. AMPK is a key regulator of energy metabolism that senses environmental or nutritional stress, as well as a potent inducer of autophagy, and thus ensures the integrity of housekeeping processes [24]. Emerging studies reveal that AMPK is an effective inhibitor of NF-kB signaling and inflammatory responses [25]. Previous research showed that the anti-inflammatory mechanism of wedelolactone was partly expressed by the down-regulation of NF-kB signaling pathways.
IFNγ was increasingly produced in the colon of DSS-treated rats that showed severe IBD like symptoms. In the colon of IFNγ rats, in contrast to wild-type (WT) mice, IP-10, MIG and MCP-1, DSS-treated WT mice exhibited a robust production of IFN-γ in the gut, a remarkable loss of body weight. It suggesting that these chemokines play important roles in the IFNγ mediated pathogenesis of DSS colitis. It is also reported that, at the initiation of DSS colitis, DSS exerts direct toxicity to the colonic epithelial cells, subsequently increasing the permeability of the intestinal mucosa and allowing transport of luminal bacterial products from the bowel lumen to submucosal tissue [26].
Interleukin-6 (IL-6)/signal transducer and activator of transcription 3 (STAT3) pathway plays essential roles in the development of inflammatory diseases including ulcerative colitis (UC). Therefore, suppression of IL-6/STAT3 signaling provides a promising therapeutic strategy in UC [27]. Moreover, wedelolactone markedly inhibited the colon tissue level of the inflammatory biomarker in colitic rats. Furthermore, wedelolactone treatment significantly inhibited DSS-induced expression of IL-6 in colon tissues. Finally, the increased phosphorylation level of STAT3, induced by DSS in experimental rats was significantly suppressed by wedelolactone. These results suggest that the inhibition of IL-6/STAT3 signaling is a potential mechanism by which wedelolactone is used in the treatment of UC.
Wencheng et.al., (2017) reported that wedelolactone treatment showed inhibitory effect on inflammatory infiltration, myeloperoxidase and alkaline phosphatase activities through MAPKs and NF-κB signaling pathways by activating AMPK on DSS induced ulcerative colitis. Similarly, wedelolactone compound significantly inhibited NOD-LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome activation and caspase-1 phosphorylation by decrease IL-1β release in DSS treated rats. The above researchers are corelated the effect of wedelolactone on AMPK-NLRP3-IL-1β signaling pathway and it plays an important role in colitis [28]. Kim et al. (2017) demonstrated that Eclipta prostrata treatment improved colitis symptoms through the modulation of immune function in intestinal epithelial cells. E. prostrata decreases the protein expressions of cyclooxygenase-2 (COX-2) and hypoxia inducible factor-1 [29].
The present study aimed to investigate whether wedelolactone effectively attenuates DSS-induced rat colitis and to explore its underlying molecular mechanism. In the current study, we found that wedelolactone treatment (50 & 100 mg/kg) significantly reversed intestinal injury and promoted the recovery of colonic epithelial cells in DSS-treated rats. STAT3 was initially identified as an acute phase response factor, an inducible DNA binding protein that binds to the IL-6 responsive element within the promoters of hepatic acute phase protein genes [30].
5. Conclusion
Our research examined the possible role wedelolactone can play in ulcerative colitis inflammation. Destruction of protective mucinous membrane resulted in epithelial cell damage and IL-6/STAT3 upregulation of inflammatory signaling pathway expression in colorectal epithelial cells leading to ulcerative colitis. The wedelolactone may contribute in down-regulating the inflammatory signaling pathway IL-6/STAT3. The IL-6/STAT3 pathway thus appeared to be a main node in the treatment of ulcerative colitis. Our results suggest that wedelolactone might be a blocking IL-6/STAT3 inflammatory signaling pathway and exhibit anti-inflammatory effect in ulcerative colitis.
Source of Funding
This work was funded by Rajiv Gandhi University of Health Sciences, Karnataka, Bangalore (Order NO: RGU: RGU/ADV.RES/GRANTS/059/2016-17).
Conflcit of interest
The authors declare no conflicts of interest.
Author contributions
Dr. Suresh Janadri contributed for investigation and analysis the markers, presented the data by using Prism software. Dr. Prakash is Project administration and writing – original draft.
Acknowledgements
Authors are grateful to Advanced Research Dept, Rajiv Gandhi University of Health Sciences, Karnataka, Bangalore for financial support. We thankful to Principal and Management, Acharya Institution, Bangalore.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Lim S., Jang H.J., Park E.H., Kim J.K., Kim J.M., Kim E.K., et al. Wedelolactone inhibits adipogenesis through the ERK pathway in human adipose tissue-derived mesenchymal stem cells. J Cell Biochem. 2012;113(11):3436–3445. doi: 10.1002/jcb.24220. [DOI] [PubMed] [Google Scholar]
- 2.Lukas M., Bortlik M., Maratka Z. What is the origin of ulcerative colitis? Still more questions than answers. Postgrad Med. 2006;82(972):620–625. doi: 10.1136/pmj.2006.047035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brain O., Travis S.P. Therapy of ulcerative colitis: state of the art. Curr Opin Gastroenterol. 2008;24:469–474. doi: 10.1097/MOG.0b013e3282ff0dd5. [DOI] [PubMed] [Google Scholar]
- 4.Xavier R.J., Podolsky D.K. Unraveling the pathogenesis of inflammatory bowel disease. Curr Pharmaceut Des. 2007;16(33):3668–3683. doi: 10.2174/138161210794079155. [DOI] [Google Scholar]
- 5.Neurath M.F. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol. 2017;14(5):269–278. doi: 10.1038/nrgastro.2016.208. [DOI] [PubMed] [Google Scholar]
- 6.Loddo I., Romano C. Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front Immunol. 2015;6:551. doi: 10.3389/fimmu.2015.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perse M., Cerar A. Dextran sodium sulphate colitis mouse model: traps and tricks. J Biomed Biotechnol. 2012;2012:718617. doi: 10.1155/2012/718617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mansoor K.P., Prakash T., Kotresha D., Prabhu K., Rama Rao N. Cerebroprotective effect of Eclipta alba against global model of cerebral ischemia induced oxidative stress in rats. Phytomedicine. 2012;19:1108–1116. doi: 10.1016/j.phymed.2012.07.004. https://doi.org/10.1016/j.phymed.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 9.Prakash T., Rama Rao N., Viswanath swamy A.H.M. Neuropharmacological studies on Wedelia calendulacea less stem extract. Phytomedicine. 2008;15:959–970. doi: 10.1016/j.phymed.2008.05.005. https://doi.org/10.1016/j.phymed.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Krawisz J.E., Sharon P., Stenson W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87(6):344–350. https://doi.org/PMID: 6092199. [PubMed] [Google Scholar]
- 11.Kawada M., Arihiro A., Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13(42):5581–5593. doi: 10.3748/wjg.v13.i42.5581. https://doi.org/10.3748/wjg.v13.i42.5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dharmani P., Leung P., Chadee K. Tumor necrosis factor-α and Muc2 mucin play major roles in disease onset and progression in dextran sodium sulphate-induced colitis. PLoS One. 2011;6(9):25058. doi: 10.1371/journal.pone.0025058. https://doi.org/10.1371/journal.pone.0025058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andujar I., Ríos J.L., Giner R.M., Miguel Cerda J., Recio Mdel C. Beneficial effect of shikonin on experimental colitis induced by dextran sulfate sodium in BALB/c mice. Evid Based Complement Alternat Med. 2012;2012:271606. doi: 10.1155/2012/271606. https://doi.org/10.1155/2012/271606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson K., Mundra H., Innis S.M. Intestinal responsiveness to experimental colitis in young rats is altered by maternal diet. Am J Physiol Gastrointest Liver Physiol. 2005;289:13–20. doi: 10.1152/ajpgi.00459.2004. https;//doi.org/10.1152/ajpgi.00459.2004. [DOI] [PubMed] [Google Scholar]
- 15.Ligumsky M., Simon P.L., Karmeli F., Rachmilewitz D. Role of interleukin 1 in inflammatory bowel disease--enhanced production during active disease. Gut. 1990;31(6):686–689. doi: 10.1136/gut.31.6.686. https://doi.org/10.1136/gut.31.6.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latz E., Xiao T.S., Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13(6):397–411. doi: 10.1038/nri3452. https://doi.org/10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coccia M., Harrison O.J., Schiering C., Asquith M.J., Becher B., Powrie F., et al. IL-1β mediates chronic intestinal inflammation by promoting the accumulation of IL-17A secreting innate lymphoid cells and CD4(+) Th17 cells. J Exp Med. 2012;209(9):595–609. doi: 10.1084/jem.20111453. https://doi.org/10.1084/jem.20111453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suwara M.I., Green N.J., Borthwick L.A., Mann J., Mayer-Barber K.D., Barron L., et al. IL-1α released from damaged epithelial cells is sufficient and essential to trigger inflammatory responses in human lung fibroblasts. Mucosal Immunol. 2014;7(3):684–693. doi: 10.1038/mi.2013.87. https://doi.org/10.1038/mi.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong F., Zhang L., Hao F., Tang H., Wang Y. Systemic responses of mice to dextran sulfate sodium-induced acute ulcerative colitis using 1H NMR spectroscopy. J Proteome Res. 2013;12(6):2958–2966. doi: 10.1021/pr4002383. https://doi.org/10.1021/pr4002383. [DOI] [PubMed] [Google Scholar]
- 20.Rot A., Von Andrian U.H. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. https://doi.org/10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 21.Greten F.R., Eckmann L., Greten T.F., Park J.M., Li Z.W., Egan L.J., et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118(3):285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Zhang D.K., Cheng L.N., Huang X.L., Shi W., Xiang J.Y., Gan H.T. Tetrandrine ameliorates dextran-sulfate-sodium-induced colitis in mice through inhibition of nuclear factor -kappaB activation. Int J Colorectal Dis. 2009;24(1):5–12. doi: 10.1007/s00384-008-0544-7. [DOI] [PubMed] [Google Scholar]
- 23.Park H.Y., Kunitake Y., Hirasaki N., Tanaka M., Matsui T. Theaflavins enhance intestinal barrier of Caco-2 Cell monolayers through the expression of AMP-activated protein kinase-mediated Occludin, Claudin-1, and ZO-1. Biosci Biotechnol Biochem. 2015;79(1):130–137. doi: 10.1080/09168451.2014.951027. https://doi.org/10.1080/09168451.2014.951027. [DOI] [PubMed] [Google Scholar]
- 24.Meley D., Bauvy C., Houben-Weerts J.H., Dubbelhuis P.F., Helmond M.T., Codogno P., et al. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem. 2006;281(46):34870–34879. doi: 10.1074/jbc.M605488200. https://doi.org/10.1074/jbc.M605488200. [DOI] [PubMed] [Google Scholar]
- 25.Salminen A., Hyttinen J.M., Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: impact on health span and lifespan. J Mol Med (Berl) 2011;89(7):667–676. doi: 10.1007/s00109-011-0748-0. https://doi.org/10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kitajima S., Takuma S., Morimoto M. Changes in colonic mucosal permeability in mouse colitis induced with dextran sulfate sodium. Exp Anim. 1999;48(3):137–143. doi: 10.1538/expanim.48.137. https://doi.org/10.1538/expanim.48.137. [DOI] [PubMed] [Google Scholar]
- 27.Li L., Shen A., Chu J., Sferra T.J., Sankararaman S., Ke X., et al. Pien Tze Huang ameliorates DSS-induced colonic inflammation in a mouse colitis model through inhibition of the IL-6/STAT3 pathway. Mol Med Rep. 2018;18:1113–1119. doi: 10.3892/mmr.2018.9051. [DOI] [PubMed] [Google Scholar]
- 28.Wei W., Ding M., Zhou K., Xie H., Zhang M., Zhang C. Protective effects of wedelolactone on dextran sodium sulfate induced murine colitis partly through inhibiting the NLRP3 inflammasome activation via AMPK signaling. Biomed Pharmacother. 2017;94:27–36. doi: 10.1016/j.biopha.2017.06.071. [DOI] [PubMed] [Google Scholar]
- 29.Kim D.S., Kim S.H., Kee J.Y., Han Y.H., Park J., Mun J.G., et al. Eclipta prostrata improves DSS-induced colitis through regulation of inflammatory response in intestinal epithelial cells. Am J Chin Med. 2017;45(5):1047–1060. doi: 10.1142/S0192415X17500562. [DOI] [PubMed] [Google Scholar]
- 30.Wegenka U.M., Buschmann J., Lütticken C., Heinrich P.C., Horn F. Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol. 1993;13(1):276–288. doi: 10.1128/mcb.13.1.276. https://doi.org/10.1128/mcb.13.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]