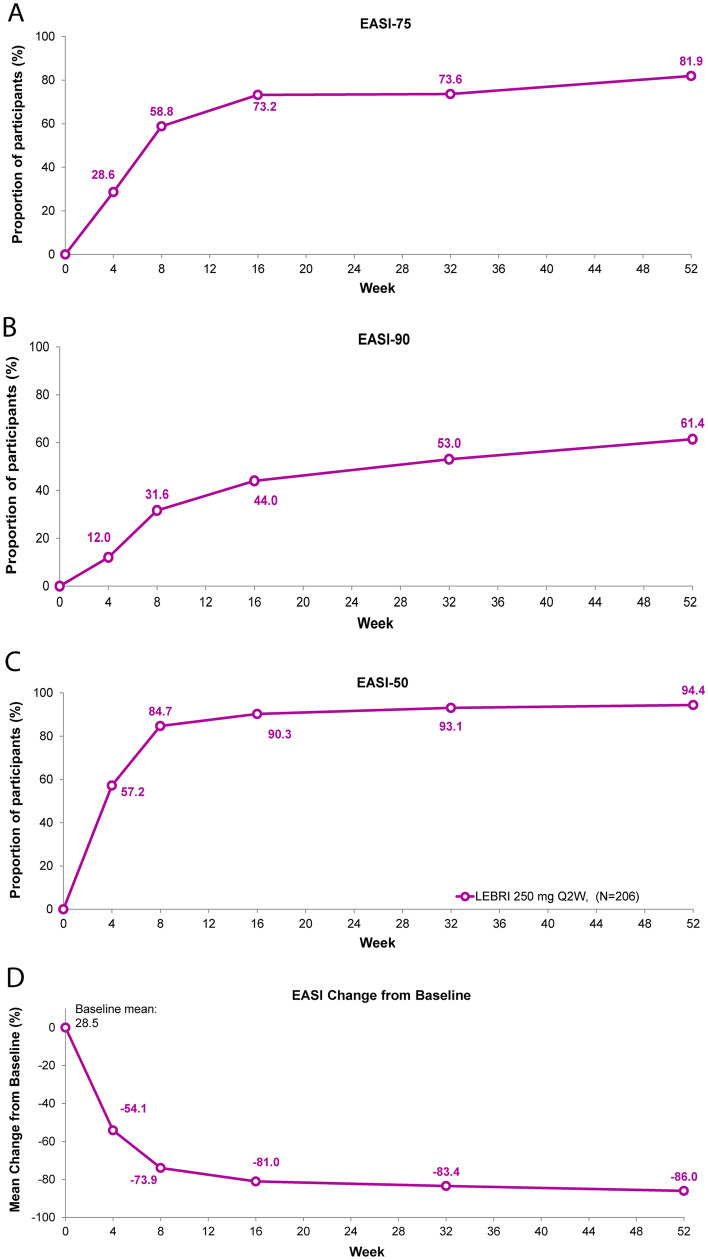
Fig. 2.
Time course response for EASI clinical outcomes. Percentage of patients (%) achieving EASI-75 (A), EASI-90 (B), EASI-50 (C), and EASI percentage change from baseline (D) through 52 weeks. Missing data due to lack of efficacy were imputed with non-responder imputation. Other missing data were imputed with multiple imputation. Abbreviations: EASI Eczema Area and Severity Index, EASI-50 50% reduction in EASI, EASI-75 75% reduction in EASI, EASI-90 90% reduction in EASI, LEBRI lebrikizumab, Q2W every 2 weeks