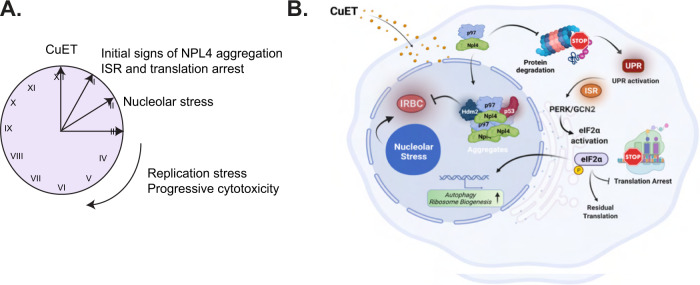
Fig. 6. Schematic illustration of the proposed model.
A Schematic presentation of the temporal order for some key cancer cell phenotypes evoked following CuET treatment. B Graphical presentation of the proposed model: the disulfiram metabolite CuET binds NPL4-p97, blocking its role in protein degradation, with ensuing proteotoxic stress and eventually inducing cell death. The NPL4/CuET-nucleated protein aggregates sequester p53 (alongside MDM2) that can no longer be targeted for degradation and thus accumulates, while the p53 activity becomes lower over time, reflecting the progressive aggregate formation and hence p53 sequestration. At the same time, CuET impact activates the cellular ISR and drives the PERK/GCN2a-mediated eIF2a phosphorylation and translational arrest. Cells respond via upregulation of genes affecting, among other mechanisms, RiBi and autophagy, two processes that, when blocked pharmacologically in combination with CuET, show more detrimental effects on cancer cell survival (Figure created with BioRender.com).