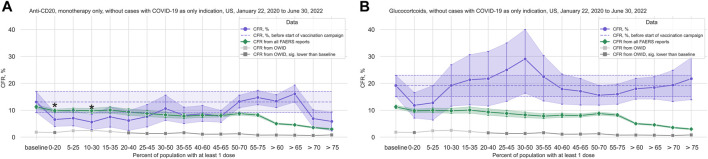
FIGURE 3.
COVID-19 CFR for the anti-CD20 and the glucocorticoid treatment groups. (A) CFR in percent computed for all cases where only treatments from the anti-CD20 group are mentioned under Suspect Product Active Ingredient (cases with COVID-19 as only indication excluded) (shown in blue), United States only, in 20% vaccination coverage bins. (B) CFR for the glucocorticoid treatment group (cases with COVID-19 as only indication excluded) (shown in blue), United States only, in 20% vaccination coverage bins. Bins are indicated as (lower bound, upper bound], and the baseline period is defined as the period before the first vaccination was administered. Since a coverage of 80% had not yet been achieved at the time of data collection, we denote bins starting from 60% or higher coverage as “> x”, indicating that these bins include all data from coverage levels higher than x. The 95% confidence intervals are estimated using bootstrap resampling, and asterisks mark data points where the CFR in the treatment group is significantly different from the CFR during the baseline period for this group (p-value from resampling, Benjamini-Hochberg with an accepted FDR of 5% over all bins and treatment groups). Data for the complete FAERS COVID-19 set are shown in green (note that significant data points are not annotated for better readability; see Figure 1A for FAERS details). The grey line shows the COVID-19 CFR from OWID data, with data points where the CFR is significantly lower than during the baseline period shown in a darker grey. Abbreviations: CFR: Case Fatality Rate; FAERS: FDA Adverse Event Reporting System; OWID: Our World In Data; sig.: Significant.