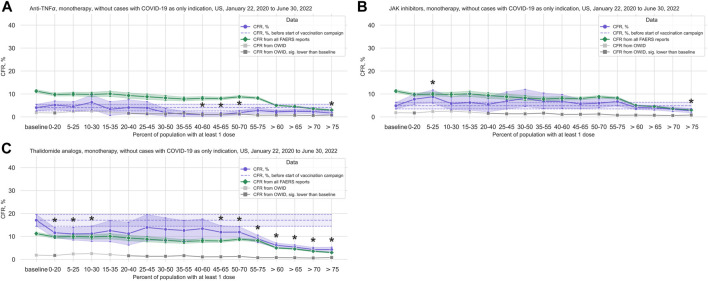
FIGURE 4.
COVID-19 CFR for the anti-TNFα, JAK inhibitors, and thalidomide analogs treatment groups. (A) CFR for the anti-TNFα treatment group (shown in blue), United States only, in 20% vaccination coverage bins. (B) CFR for the JAK inhibitors treatment group (shown in blue), United States only, in 20% vaccination coverage bins. (C) CFR for the thalidomide analogs treatment group (shown in blue), United States only, in 20% vaccination coverage bins. All treatment groups are monotherapy only, and cases where COVID-19 is the only indication are excluded. Bins are indicated as (lower bound, upper bound], and the baseline period is defined as the period before the first vaccination was administered. Since a coverage of 80% had not yet been achieved at the time of data collection, we denote bins starting from 60% or higher coverage as “> x”, indicating that these bins include all data from coverage levels higher than x. The 95% confidence intervals are estimated using bootstrap resampling, and asterisks mark data points where the CFR in a treatment group is significantly different from the CFR during the baseline period for this group (p-value from resampling, Benjamini-Hochberg with an accepted FDR of 5% over all bins and treatment groups. Data for the complete FAERS COVID-19 set are shown in green (note that significant data points are not annotated for better readability; see Figure 1A for FAERS details). The grey line shows the COVID-19 CFR from OWID data, with data points where the CFR is significantly lower than during the baseline period shown in a darker grey. Abbreviations: CFR: Case Fatality Rate; FAERS: FDA Adverse Event Reporting System; OWID: Our World In Data; sig.: Significant.