Version Changes
Revised. Amendments from Version 1
In response to reviewers comments we have made following changes We have updated the abstract section as recommended by the reviewers. In the Introduction section, we have replaced “ITNs” by “LLINs” in all paragraphs. In the fourth paragraph, “the country” has been replaced by “Côte d’Ivoire”. We have made a change in the penultimate sentence of this paragraph for better understanding. In the last paragraph, a new sentence has been added. In the methods section, notably in the Mosquito strain part, we have replaced “senso” by “sensu” as recommended by the reviewers. In the Laboratory resistance selection part, we have rephrased sentences and added the insecticide concentration, in the second paragraph. In the third paragraph, the exposure time has been added and we have replaced “Permanent” by “Permanent”. We have updated also the fourth paragraph. We have revised some sentences in Bioassays, Molecular analysis, and Statistical analysis parts. In the results section, we have replaced “Overall” by “By contrast” at beginning of the last paragraph of “Change in mosquito mortality and susceptibility to pyrethroid during the selection” part. In the discussion section, we have added new references in the second paragraph as recommended by the reviewers therefore all the reference numbers that follow have changed. We have also replaced “pippiens” by “pipiens” in whole the text. We have added sentence, replaced “programmers” by “programmes” and deleted “or stop” in the last paragraph. In Data availability section, we have provided new DOI In References part, reference 37 and 38 have been added.
Abstract
Background: The indiscriminate use of insecticides in agriculture and public health lead to a selection of resistance mechanisms in malaria vectors compromising vector control tools and strategies. This study investigated the metabolic response in the Vgsc-L995F Anopheles gambiae Tiassalé resistance strain after long-term exposure of larvae and adults to deltamethrin insecticide.
Methods: Vgsc-L995F An. gambiae Tiassalé strain larvae were exposed over 20 generations to deltamethrin (LS) and adults to PermaNet 2.0 (AS) and combining exposure at larvae and adult stages (LAS) and compared to unexposed (NS) group. All four groups were subjected to the standard World Health Organization (WHO) susceptibility tube tests using deltamethrin (0.05%), bendiocarb (0.1%) and malathion (5%). Vgsc-L995F/S knockdown-resistance ( kdr) mutation frequency was screened using multiplex assays based on Taqman real-time polymerase chain reaction (PCR) method. Additionally, expression levels of detoxification enzymes associated to pyrethroid resistance, including CYP4G16, CYP6M2, CYP6P1, CYP6P3, CYP6P4, CYP6Z1 and CYP9K1, and glutathione S-transferase GSTe2 were measured.
Results: Our results indicated that deltamethrin resistance was a response to insecticide selection pressure in LS, AS and LAS groups, while susceptibility was observed in NS group. The vectors showed varied mortality rates with bendiocarb and full susceptibility to malathion throughout the selection with LS, AS and LAS groups. Vgsc-L995F mutation stayed at high allelic frequency level in all groups with a frequency between 87% and 100%. Among the overexpressed genes, CYP6P4 gene was the most overexpressed in LS, AS and LAS groups.
Conclusion: Long-term exposure of larvae and adults of Vgsc-L995F resistant- An. gambiae Tiassalé strain to deltamethrin and PermaNet 2.0 net induced resistance to deltamethrin under a significant effect of cytochromes P450 detoxification enzymes. These outcomes highlight the necessity of investigating metabolic resistance mechanisms in the target population and not solely kdr resistance mechanisms prior the implementation of vector control strategies for a better impact.
Keywords: Resistance selection, Anopheles gambiae, Deltamethrin, PermaNet 2.0, Tiassalé strain, P450 genes, Vgsc-L995F, Côte d’Ivoire
Introduction
Insecticides remain the key components of malaria vector control in Africa, used to treat bed nets and for indoor residual spraying (IRS). Intervention programs based notably on long-lasting insecticide-impregnated bednets (LLINs) have recently contributed to the reduction of malaria morbidity and mortality in sub-Saharan Africa by 62% between 2000 and 2019 1 . Unfortunately, the extensive use of insecticides in public health has subjected malaria vectors to enormous selective pressure, leading to the development of insecticide resistance, which itself leads to the loss of effectiveness of vector control tools 2 . Many studies have reported vectors resistance to the main insecticide classes used in vector control programs 3– 6 .
Insecticide resistance is described as the ability of insects to survive and adapt to the effects of an insecticide 7 . Because of their knockdown effect for mosquitoes and low toxicity to humans, pyrethroids were the only recommended class of insecticides used in insecticide-treated nets (LLINs) until 2019 8 . Recently, several LLINs with new chemicals such as pyriproxyfen and Chlorfenapyr, pre-qualified by the World Health Organization (WHO) have been developed and are currently deployed in Africa 8– 10 . However, although these LLINs still contain pyrethroid insecticides, their efficacy against resistant mosquitoes is reinforced by the presence of a second chemical, which is either a synergist or an insecticide.
In Africa, pyrethroid resistance has been linked mainly to two resistance mechanisms: target site resistance and metabolic resistance. Target site resistance involves non-synonymous mutations in the gene encoding the voltage-gated sodium channel ( Vgsc) present in the central nervous system of mosquitoes, which is a functional target of pyrethroid and DDT insecticides. They are often referred to as “knockdown resistance” ( kdr) because of the ability of insects possessing these alleles to be resistant to the insecticide’s knockdown effect. Kdr is conferred by two mutations occurring at the same position Vsgc-995F/S in the major African malaria vector An. gambiae 11 . Both mutations have been reported to be widely distributed in Anopheles populations in sub-Saharan Africa 12– 14 . The metabolic resistance is caused by the elevated level of detoxification enzyme activity and relates to metabolizing, which increases the metabolism or sequestration of the insecticide before it reaches its target site in mosquitoes 15, 16 . Three major families of detoxification enzymes which are linked to insecticide resistance include cytochrome P450 (P450 or CYP) monooxygenases, carboxyl/choline esterases (CCEs), and glutathione- S-transferases (GSTs). Several studies have reported these detoxification enzymes are related to pyrethroid resistance in malaria vector 2, 17, 18 . Other mechanisms of resistance involve cuticular thickening and overexpression of chemosensory protein 19, 20 . The evolution of the pyrethroid resistance phenomenon and the multiple mechanisms of resistance represent a great challenge that requires attention.
In addition to the use of insecticides in public health, several studies have reported that the indiscriminate use of pyrethroids and other classes of insecticides for agricultural purposes has increased selection pressure for resistance in mosquito populations in Africa 5, 6, 12, 21, 22 . Due to the growing population in Africa, the use of insecticides is becoming a key element in increasing agricultural yields 23 . In West Africa, three countries including Côte d'Ivoire, Ghana, and Nigeria were the largest pesticide importer between 2005 and 2015 24 . Pyrethroids appeared to be the most popular insecticides used, accounting for 90% of all reported insecticides used in the southern part of Côte d’Ivoire. The use of insecticides in areas of intensive agriculture often results in the contamination of mosquito larvae breeding sites 21 . The increasing use of pesticides in agricultural settings was also reported to influence insecticide resistance across Côte d'Ivoire. Anopheles gambiae s.l larvae from the Tiassalé rice field were resistant to the four "classical” families of insecticide used in public health. Therefore, environmental parameters, such as the presence of xenobiotic compounds, influence both the overall reaction of mosquitoes to pyrethroids and the selection of resistance mechanisms 5, 21, 25, 26 .
Currently, the use of long-lasting insecticidal nets (LLINs) and pesticides for public health and agricultural purposes are incriminated as a source of resistance selection in mosquitoes, as it relies heavily on pyrethroid insecticides. However, the relative contribution of target site resistance and metabolic resistance mechanisms in the selection of pyrethroid resistance in Anopheles mosquitoes remains unclear. In addition, few studies have examined the impact of metabolic resistance in the selection of pyrethroid resistance in kdr-resistant An. gambiae. This study aimed to determine how much a pyrethroid phenotypic resistance can be driven by metabolic mechanism in An. gambiae mosquitoes. To reach this, we performed a resistance selection experiments with Vgsc-L995F resistant An. gambiae Tiassalé strain both at larvae and adult stages and determine underlying metabolic resistance mechanisms. We purposely used a high Vgsc-L995F-resistant An. gambiae colony to detect only an induced metabolic effect on mosquito phenotype.
Methods
Mosquito strain
This study was conducted with laboratory-reared colonies ( An. gambiae sensu stricto specimens) isolated from the pyrethroid-resistant An. gambiae s.l mosquitoes collected in the irrigated rice field of Tiassalé, southern Côte d’Ivoire 27 . The locality of Tiassalé is characterized by an intensive use of pyrethroid and neonicotinoid insecticides in agriculture ( e.g. irrigated rice) 21, 28 . Earlier studies have shown that An. gambiae s.l from Tiassalé carry the kdr and Ace1 point mutations as well as P450 genes conferring resistance to pyrethroids, DDT, carbamates and organophosphates 13, 25, 26, 29 . The mosquito colony used in the present experiments was derived from the An. gambiae Tiassalé strain and maintained in the insectary of Centre Suisse de Recherche Scientifiques en Côte d'Ivoire (CSRS) over several generations since 2010, without being subjected to any insecticide selection pressure. Prior to start the selection the Vgsc-L995F allele mutation was confirmed in the Tiassalé An. gambiae strain using TaqMan RT-PCR method as described below.
Laboratory resistance selection
The experiments used four colonies derived from the same parental population ( i.e., G0 parent generation) of An. gambiae Tiassalé strain carrying Vgsc-L995F kdr mutation:
-
-
Larvae-Selected (LS) group: colony selected with deltamethrin at larval stage over 20 generations;
-
-
Adult-Selected (AS) group: colony selected with PermaNet 2.0 LLIN at adult stage over 20 generations;
-
-
Larvae+Adult Selected (LAS) group: colony selected at both larvae and adult stages with deltamethrin and PermaNet 2.0 respectively and
-
-
Non-Selected (NS) group: colony non-subjected to any insecticide resistance selection.
The LS group mosquitoes were selected with a solution of deltamethrin insecticide prepared from a stock solution containing 100mg of deltamethrin (100%) powder in 1000 mL of water (10% or 100mg.L). We exposed only stage II larvae of each generation up to 20 generation for 24 hours to a sub-lethal dose of deltamethrin inducing 20% mortality (LD 20), in order to minimize excessive losses while exerting resistance selection. We determined the LD 20 with 100 larvae distributed per batch of 25 larvae and exposed to deltamethrin for 24 hours in plastic cups containing 100 mL of water and fed with 0.075g powdered Friskies cat food. To maintain the selection pressure, a new LD 20 was determined after every five generations (G1, G6, G11, G16). The corresponding doses were 5, 6, 14 and 18 mL respectively. The different LD 20 were determined by counting larvae survival and using PoloPlus 1.0 software (software using probit or logit regression analyses). Prior to each exposure, the deltamethrin solution (10%) was prepared 24 hours in advance to allow complete dilution of the deltamethrin powder in water. After exposure, all surviving larvae were transferred to clean water without insecticide, fed and allowed to emerge. At each generation, two- to five-day old female adult were blood-fed to obtain the next generation. We conducted the rearing at the CSRS insectary under standard conditions (26 °C ± 2 °C, 75% ± 10% and a photoperiod of 12:12 h).
In the AS group, the selection was conducted by exposing two- to five-day-old, non-blood fed adult female mosquitoes to new PermaNet 2.0 LLIN. PermaNet 2.0 LLIN was obtained from the National Malaria Control Program (NMCP). PermaNet 2.0 LLIN is coated with a deltamethrin concentration of 55 mg/m 2. The long-term resistance selection was conducted by exposing adults of each generation to a PermaNet 2.0 sub-lethal time causing 20% (LT 20) mortality. To determine the LT 20, we exposed four cohorts of 100 females at different set times in 15×15×15cm holding cages covered with PermaNet 2.0 LLIN. After every five generations (G1, G6, G11, G16) the following LT 20s 15, 17, 35 and 45 min were respectively determined to continue the selection. After exposure, the mosquitoes were transferred to new cages covered with a non-treated net and the mortality was recorded 24 hours later. The LT 20 was determined by counting the number of dead mosquitoes and using the PoloPlus software as describe above. We determined the LT 20 before starting the experiment and at each series of five generations. At each generation, mosquitoes were all transferred to the same holding cage after exposure to LLIN and fed with sugar solution diluted at 10%. Mosquitoes were blood-fed after 24 hours post-exposure in order to perform an insectary rearing. The AS group was reared in the CSRS insectary in the similar standard conditions (26°C ± 2°C, 75% ± 10% and a photoperiod of 12:12 h) described for LS group above.
The LAS group mosquitoes were selected over several generations (G1, G6, G11, G16) by exposing larvae first to deltamethrin (5, 6, 14 and 18 mL) then waiting up to emergence before exposing adults to PermaNet 2.0 LLIN (15, 20, 40 and 60 min) as describe above.
The NS group was reared without any selection.
Bioassays
To determine the phenotypic resistance within each selected group ( i.e., LS, AS and LAS) or non-selected group ( i.e., NS), susceptibility tube tests with discriminating doses of deltamethrin (0.05%), bendiocarb (0.1%) and malathion (5%) using treated papers sourced from WHO according to WHO protocol 7 were performed. For each insecticide, four batches of 20–25 two- to five-day old female mosquitoes from the three selected groups and non-selected group were exposed to insecticide-impregnated papers in the presence of two negative control batches. Controls included batches of mosquitos from each group and exposed to untreated paper. The number of mosquitoes knocked out was recorded every five-minute intervals during the one-hour exposure. After exposure, tested mosquitoes were transferred into a holding tube and fed with 10% honey. Mortality was recorded 24 hours post-exposure. For each group, susceptibility assays were performed every fifth generation (G0, G5, G10, G15, G20).
Mosquito sample preparation
After recording the 24-hour mortality, mosquitoes were killed by immersing them into absolute ethanol. They were later placed on filter papers to remove the extra ethanol before being transferred gently into a 1.5 mL microcentrifuge tube that contained RNAlater (Ambion, Inc., Austin, Texas, US). Then, we stored the mosquitoes at 4°C overnight to allow complete penetration of the product into the tissues. Exposed and negative control mosquitoes were stored separately. After one day, the excess RNAlater was removed and the tubes containing the mosquitoes were stored at -20°C until DNA and RNA extraction.
DNA and RNA extraction
For each of the four mosquito groups, DNA and RNA extractions were performed with 30 individual mosquitoes randomly selected from the G0, G10 and G20 generations to detect kdr mutation genes whereas for the detection of detoxification enzymes, the target genes expression levels were measured with 50 individuals split into pools of 10 mosquitoes for the G0 and G20 generations only per group (LS, AS, LAS and NS).
DNA and RNA were extracted using the MagnaMedics magnetic bead kit (MagnaMedics GmbH, Aachen, Germany). Both individual and batch mosquitoes were ground in 200 µL TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0) per tube. Then 150 µL of lysis buffer was added, mixed for 15 s with a vortex before incubating for 10 min at room temperature with intermittent vortexing every 2 min for 15 s. After incubation, the mixture was spun by centrifugation at 16,000 ×g for 2 min. The supernatant was transferred to a new 1.5 mL tube, and then, 20 µL of magnetic beads and 440 µL of binding buffer were added and vortexed for 15 s. The mixture was incubated for 10 min as described above. To allow sedimentation of the magnetic beads, the tubes were placed on a magnetic rack for 2 min and the supernatant was discarded. The 200 µL of wash buffer was added twice to wash the beads while mixing and placed the tubes again for 2 min on the magnetic rack. Following the extraction, 180 µL of elution buffer to extract the nucleic acids were added and incubated for 10 min in a water bath at 50°C and vortexed in between as described above. After the incubation, the mixture was spun and placed on the magnetic rack. Finally, the supernatant that contained the purified DNA and RNA was transferred the new 1.5 mL tubes and kept at 80°C.
Molecular analysis
The multiplex TaqMan RT-PCR methods was used to detect two kdr point mutations in the voltage-dependent sodium channel ( i.e., Vgsc-L995F/S) from individual mosquitoes using the protocol developed by Bass et al. 30 as adapted by Mavridis et al. 31 ( Table 1).
Table 1. List of primers and probes used for molecular analysis.
| Reagent | Oligo_Name | Sequence (5' - 3') | Assay description
Green: FAM Yellow: HEX Red: Atto647N |
Optimized
Concentration (nM) |
|
|---|---|---|---|---|---|
| Primer | kdr_F | CATTTTTCTTGGCCACTGTAGTGAT | kdr_Vgsc-L995F/S | none | 500 |
| Primer | kdr_R | CGATCTTGGTCCATGTTAATTTGCA | kdr_Vgsc-L995F/S | none | 200 |
| Probe | kdr-wt_P | CTTACGACTAAATTTC | wild type - kdr_Vgsc-L995 | HEX | 500 |
| Probe | kdrW-mt_P | ACGACAAAATTTC | mutant - kdr-west_Vgsc-995F | FAM | 500 |
| Probe | kdrE-mt_P | ACGACTGAATTTC | mutant - kdr-east_Vgsc-995S | Atto647N | 500 |
| Primer | RPS7_Fj | CCACCATCGAACACAAAGTTGA | RPS7-Detox (A-D) | none | 100 |
| Primer | RPS7_R | TGCTGCAAACTTCGGCTATTC | RPS7-Detox (A-D) | none | 200 |
| Probe | RPS7_P1 | CCGTGACGTTACGTTCGAATTCCCA | RPS7-Detox (A-D) | FAM | 250 |
| Primer | P3_Fj | ACAATGTGATTGACGAAACCCT | CYP6P3-Detox (A) | none | 400 |
| Primer | P3_R | GGATCACATGCTTTGTGCCG | CYP6P3-Detox (A) | none | 500 |
| Probe | P3_P1 | ACCCGCGTACCGTCTGTGGACT | CYP6P3-Detox (A) | HEX | 350 |
| Primer | M2_F2 | CTGGCGTTGAATCCAGAGGT | CYP6M2-Detox (A) | none | 600 |
| Primer | M2_Rj | GATACTTGCGCAGTGATTCATTAAG | CYP6M2-Detox (A) | none | 400 |
| Probe | M2_P | AGAGAAATCCTGCAAAAGCACAACGGAGA | CYP6M2-Detox (A) | Atto647N | 250 |
| Primer | K1_F | CCGACACGTGGTGATGGATAC | CYP9K1-Detox (B) | none | 200 |
| Primer | K1_Rj | CGTCGTCGGTCCAGTCAAC | CYP9K1-Detox (B) | none | 400 |
| Probe | K1_P | CAATCTTCTGATGCAGGCCCGCAA | CYP9K1-Detox (B) | HEX | 300 |
| Primer | P4_Fj | CTGGACAACGTTATCAATGAAACC | CYP6P4-Detox (B) | none | 400 |
| Primer | P4_R | GCACGGTGTAATCACGCATC | CYP6P4-Detox (B) | none | 500 |
| Probe | P4_P | CCGATCGAGTCACTTTCGCGCG | CYP6P4-Detox (B) | Atto647N | 300 |
| Primer | Z1_Fj | CCCGCAACTGTATCGGTCTG | CYP6Z1-Detox (C) | none | 100 |
| Primer | Z1_R | TTCGGTGCCAGTGTGATTGA | CYP6Z1-Detox (C) | none | 600 |
| Probe | Z1_P1 | TGATGCTGTCCCGATTTAACTTTTCGGC | CYP6Z1-Detox (C) | HEX | 250 |
| Primer | TE2_Fj | CCGGAATTTGTGAAGCTAAACC | GSTE2-Detox (C) | none | 100 |
| Primer | TE2_R | GCTTGACGGGGTCTTTCGG | GSTE2-Detox (C) | none | 400 |
| Probe | TE2_P | CGGTACGATCATCACCGAGAGCCAC | GSTE2-Detox (C) | Atto647N | 300 |
| Primer | P1_Fj | ACAGGTGGTGAACGAAACCC | CYP6P1-Detox (D) | none | 100 |
| Primer | P1_R | GGTGTAATCCTGTCCCGCAA | CYP6P1-Detox (D) | none | 500 |
| Probe | P1_P | CCGCTCGAAACGACGCTGCG | CYP6P1-Detox (D) | HEX | 300 |
| Primer | G16_Fj | GTCCAAGAAGTTGCGTCGGAC | CYP4G16-Detox (D) | none | 200 |
| Primer | G16_R | TCTTCGATTTGCGTTGACGTG | CYP4G16-Detox (D) | none | 200 |
| Probe | G16_P1 | CTGCAGGCCGACATCATTTTGAAGC | CYP4G16-Detox (D) | Atto647N | 300 |
We measured the expression level of detoxification enzymes including CYP4G16, CYP6M2, CYP6P1, CYP6P3, CYP6P4, CYP6Z1, and CYP9K1, and the glutathione S-transferase GSTe2. For this step, we used the quantitative RT-PCR methods described by Mavridis et al. 32 . We measured the expression levels of the detoxification enzymes as well as the reference gene coding for the ribosomal protein S7 (RPS7). All RT-(qPCR) reactions were performed in 10 µL volumes including 1 µL of DNA/RNA, 9 µL of master mix comprising primers and probes. The final concentration of primers and probes is shown in Table 1. The reagents used in this study were provided by Fast-Track Diagnostics (FTD, Esch-sur-Alzette, Luxembourg). All reactions RT-PCR and RT-qPCR were performed in 96-well plates (Sarstedt, Nümbrecht, Germany; catalog number: 72.1980.202) on a C1000 touch CFX96 TM real-time PCR system (Bio-Rad Laboratories, Hercules, CA, US). The thermal cycler parameters were as follows; reverse transcription at 50°C for 15 min, RTase inactivation and initial denaturation for 3 min at 95°C, having 40 cycles of denaturation for 3 s at 95°C and extension step at 60°C for 30 s.
Statistical analysis
Mortality results during susceptibility tests were interpreted according to WHO criteria 7 : a mortality below 90% suggests resistance, equal to or greater than 98% indicates susceptible; and between 90% and 97% suggests a possibility of resistance that needs to be confirmed. We performed the survival analysis to visualize the time of pyrethroid knockdown by comparing the LS, AS, LAS and NS groups to the parent population (G0).
The expression level of genes determined at G0, G10 and G20 for LS, AS, LAS and NS groups were analyzed using the Pfaffl 33 method implemented in the genetic analysis software REST 2009 version v2.013. This software allowed to enter the Threshold Cycle (C T) values of the genes detected at G0, G10 and G20 generations. We calculated the fold change of each gene of interest relative to the parental G0 generation with 95% confidence intervals and P-values within each group. The C T values of the reference gene coding for the ribosomal protein (RPS7) and of each target gene for each generation were re-assigned jointly to G10 and G20 (with or without insecticide selection) and G0 which represented the parent population. The fold changes were calculated based on the average values after 2,000 iterations.
Statistical analyses were performed with R software version 4.0.3 34 using RStudio version 1.3.1093 35 . The R package tidyverse was used for data sorting, manipulation and visualization. We used the R packages survival and survminer to plot the survival curves and calculated the Kaplan-Meier estimates 36 with as significance level set at α = 0.05. We conducted a Chi-square test to compare the significance level of the mortalities between each of the three LS, AS and LAS and groups and NS group.
Results
Knockdown time
The knockdown effects varied substantially across generations within the LS, AS, LAS and NS groups ( Figure 1).
Figure 1. Kaplan-Meier survival curves showing the cumulative survival over the 60 minutes exposure to diagnostic concentrations of deltamethrin (0.05%) in the WHO insecticide susceptibility assay.
The P-values represent the Log-rank test p-value. ( A) NS-LS; ( B); NS-AS; ( C) NS-LAS.
The knockdown rate observed in LS group ( Figure 1A) ranged from 13% to 100%. The highest rate was obtained at G0 (parent population) after 50 min while the lowest was found at the end of the one-hour exposure time with G20 adults. The insecticide knocked down more than 50% of the parent mosquitoes (G0) after 25 min of exposure. After five generation of selection, time allowing 50% knockdown was estimated at 45 min before reaching a maximum kdt 50 of 55 min at G15. However, it was not possible to determine kdt 50 for G20 because corresponding knockdown rates were less than 15% at any time point during exposure.
In the AS group ( Figure 1B), the knockdown rate declined from 100% (G0) to 13% at G20 as observed in the LS group. However, kdt 50 was estimated at 60 min and observed early after five generations of selection while it was not determined there from G15.
In the LAS group ( Figure 1C), the knockdown rate dropped to 8% at G20 while it was 100% at G0. However, no kdt 50 was determined at any generation except at G0.
In the NS group, the knockdown rate was kept very high for all generations comparing to LS, AS, and LAS ( Figure 1A, B, C). The kdt 50 was recorded much earlier (between 15 and 25 min) during the exposure without substantial variations among the generations.
Change in mosquito mortality and susceptibility to pyrethroid during the selection
Mortalities observed in controls were less than 5% for each WHO tube test performed, confirming the validity of the test. Figure 2 shows the WHO susceptibility test data in LS, AS, LAS and NS groups during the selection process. Mortality with deltamethrin, which was 85% in parent population G0, considerably varied among LS, AS, LAS and NS groups and across generations.
Figure 2.
Dynamics of mortality with bendiocarb (0.1%), deltamethrin (0.05%), malathion (5%) in An. gambiae Tiassalé divided into three groups ( A) LS, ( B) AS, ( C) LAS and ( D) NS. Error bars indicate 95% confidence intervals. The mortality threshold of 98% indicates full susceptibility according to WHO criteria 7 .
Indeed, in the selected LS, AS and LAS groups ( Figure 2A, B, C), deltamethrin mortality dropped significantly from G0 to G20 (P < 0.001). Within LS group, deltamethrin mortality rate decreased significantly (X 2 = 94.5; df =1; P < 0.001) to 14.9% at G20 with a resistance factor of 5.7 ( Figure 2A). The mortality in LS group started to differ significantly (X 2 = 77.3; df = 1; P < 0.001) after 10 generations. Additionally, mortality decreased significantly (X 2 = 83.1; df = 1; P < 0.001) to 20.2% at G20 in AS group ( Figure 2B) with a resistance factor of 2.1 and a significant difference (X 2 = 8.1; df = 1; P < 0.01) observed just after five generations. The greatest resistance factor (8.1%) was found in the LAS group with mortality declining to 10.9% at G20 ( Figure 2C). A significant difference (X 2 = 19.6; df = 1; P < 0.001) was observed as early as G5.
In the NS group ( Figure 2C), deltamethrin mortality increased progressively, and reached a rate of 100% at G20 ( Figure 2D). Deltamethrin mortality in NS group was significantly higher compared with the three LS group (X 2 = 137.4; df = 1; P < 0.001), AS group (X 2 = 125.2, df = 1, P < 0.001) and LAS group (X 2 =150.9, df = 1, P < 0.001).
By contrast, very high mortalities (100%) were observed in the LS, AS, LAS and NS groups at any generations ( i.e., from G0 to G20) with both bendiocarb and malathion, thus suggesting full susceptibility of all the four mosquito groups to both insecticides ( Figure 2A, B, C, D).
Allelic frequency
The target site resistance gene Vgsc-L995F remained detectable at a high allelic frequency in the three selected groups (LS, AS, and LAS) as well as in non-selected groups (NS) ( Figure 3). However, the mutant Vgsc-L995S allele was not found in any groups. Vgsc-L995F allelic frequency remained the same from G0 to G20 in the LS group ( Figure 3A), but there was a slight decrease in the frequencies observed in the AS ( Figure 3A), LAS ( Figure 3B) and NS ( Figure 3C) groups. In the NS group, the frequency of Vgsc-L995F declined from 100% at G0 to 85% at G10, before rising to 93% at G20.
Figure 3. Dynamics of target site mutation Vgsc-L995F through resistance selection.
( A) LS group; ( B) AS group; ( C) LAS group and ( D) NS group. Percentages indicate the frequency of resistance alleles while bars indicate actual numbers. For each group, the Vgsc-L995F mutation was genotyped in individual mosquitoes.
Metabolic enzyme
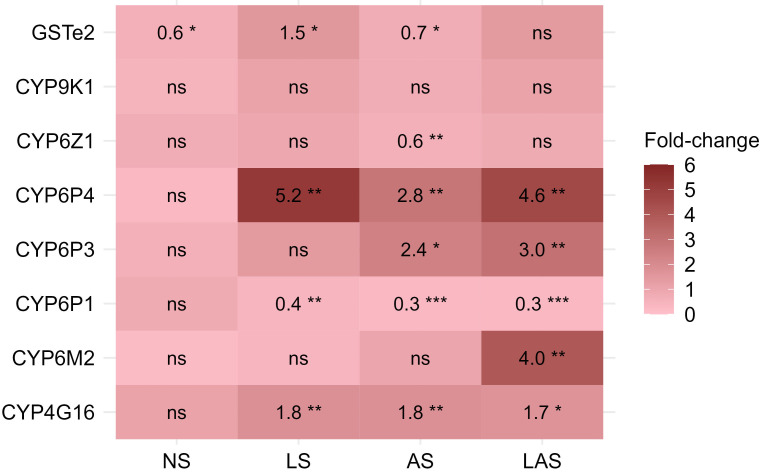
Among the eight detoxification genes, the CYP6P4, CYP4G16, and glutathione S-transferase (GSTe2) were found to be overexpressed in LS group, while the CYP6P3, CYP6P4 and CYP4G16 were found overexpressed in AS group and the CYP6P3, CYP6P4, CYP6M2 and CYP4G16 were overexpressed in LAS group, when comparing G20 to G0 ( Figure 4). In contrast, none of the eight genes were overexpressed in the NS group at G20. The CYP6P4 was the most overexpressed gene with 5.2-fold, 2.8-fold and 4.6-fold higher up at G20 compared to the G0 parent in the three selected LS, AS and LAS groups respectively. The CYP4G16 gene, responsible for cuticular resistance, was detected, up-regulated about two-fold higher at G20 compared with G0 (P < 0.01) in the three selected groups. In the AS group, we found CYP6P3 gene significantly overexpressed with an up-regulation up to 2.4-fold compared to G0 (P < 0.05). The glutathione S-transferase GSTe2 was overexpressed about 1.5-fold higher in the LS group at G20 in comparison with G0 (P < 0.05). CYP6M2 gene associated to pyrethroid resistance was found as the second gene significantly overexpressed with 4-fold higher at G20 compared to G0 in LAS group.
Figure 4. Differential expression levels for putative detoxifying genes measured in selected groups.
Fold changes give the change in expression level of mosquito populations at the 20th generation relative to the reference generation (parent G0). Fold changes were estimated using REST2009 analysis software for each gene. ns: not significant; * p < 0.05; ** p < 0.01; *** p < 0.001.
Discussion
The current study assessed the dynamics of insecticide resistance mechanism across different generations of An. gambiae carrying Vgsc-L995F exposed to deltamethrin. The outcomes showed that repeated exposure of An. gambiae larvae and adults to deltamethrin maintained the resistance mutation Vgsc-L995F allele at a high level, alongside the selection for detoxification genes. This selection of metabolic resistance genes was associated with pyrethroid resistance that induced a significant reduction in the susceptibility and mortality of An. gambiae Tiassalé strain exposed to deltamethrin across generations.
In fact, the phenotypic resistance, associated with a drastic reduction in mosquito mortality, increased significantly after only a few generations during selection either at larval or adult stages, and both at larval and adult stages concomitantly (G5) compared to non-selected group. This suggests that the increase in mosquitoes’ resistance results from selection pressure exerted by repeated exposure to a chemical insecticide either directly in their environment at larval stage 37 or adult stage through contact with a treated surface such as bed net across generations 38 . Similarly, recent laboratory studies have shown that An. coluzzii mosquitoes gain resistance when subjected to continuous selection pressure but loose this resistance in the absence of insecticide exposure after 15 generations 39 .
The frequency of Vgsc-L995F allele stayed at a very high level in the selected and non-selected groups throughout the selection, suggesting the increased level of phenotypic resistance to deltamethrin in our context is due to the involvement of metabolic resistance as shown the overexpression of some detoxification genes. Earlier studies in An. gambiae and Culex pipiens mosquitoes 39, 40 reported that in a population where the kdr mutation is fixed or absent, phenotypic resistance could be associated with a metabolic resistance mechanism. On the other hand, the phenotypic resistance is lost if the selection pressure is not maintained; this was the case in our non-selected group where susceptibility test did not reveal any apparent resistance. Our data are consistent with studies conducted on Aedes aegypti in which significant loss of resistance was observed in the absence of selection pressure over 10 generations 41 . These results also suggest that under field conditions, resistance can decline without insecticide pressure, likely due to the fitness costs associated with insecticide resistance.
In response to resistance selection with deltamethrin directly by exposure of larvae to deltamethrin or adults through PermaNet 2.0 LLIN or by combined exposure (larvae and adult stages), the P450 enzymes including CYP6M3, CYP6M2, CYP6P4, CYP4G16 and the glutathione S-transferase GSTe2 were overexpressed. The overexpression of four P450 genes confirms their roles in insecticide detoxification as they have previously been associated with pyrethroid resistance in Anopheles species 42 , as reported in Tiassalé 13 strain 43 and in An. arabiensis from Ethiopia, including the GSTe2 44 . Indeed, in the present experiments, detoxification enzyme activity increased significantly in the LS, AS and LAS groups after 20 generations of selection from the parent G0 individuals having the kdr-L995F mutation. In contrast, no increase in detoxification gene activity was observed in the NS group. This observation could suggest that the metabolic resistance has strengthened under increasing selection pressure and played an important role in the development of high levels of resistance. A similar study examining pyrethroid resistance selection in Culex pipiens pallens mosquitoes found that the kdr mutation and metabolic genes contribute to pyrethroid resistance but play different roles under low and high selection pressure 40 .
The CYP6P4 gene was the most overexpressed enzyme in LS, AS and LAS groups. The fact that this gene was the most overexpressed might imply that it plays a key role in the metabolic resistance against deltamethrin. This cytochrome P450 was detected in wild pyrethroid-resistant An. coluzzii mosquito populations collected in irrigated rice fields treated with pesticides ( e.g., pyrethroid, organophosphate, triazine, carbamate, neonicotinoid) in Tiassalé and southern region of Côte d'Ivoire 26, 38 . Another earlier and previous study has demonstrated that CYP6P4 is the major P450 involved in pyrethroid resistance in kdr-free An. arabiensis in Chad 17 . Therefore, further studies are needed to better understand the role of CYP6P4 in deltamethrin metabolism in the An. gambiae. In addition, CYP6P3 and CYP6M2, found to be associated with pyrethroid resistance 45, 46 and CYP4G16 involves in cuticular resistance 47 , were significantly overexpressed in LS, AS and LAS groups respectively. This could explain the low mosquito mortality to deltamethrin in the LS, AS and LAS groups observed in the present laboratory studies. Similarly, a recent laboratory selection study detected overexpression of these enzymes in the laboratory-reared resistant strain An. gambiae Tiassalé 13 and VK7 2014 strains subjected to selection pressure with WHO paper impregnated with deltamethrin 0.05% 43 . Moreover, GSTe2 was found as an overexpressed detoxification gene in An. gambiae Tiassalé 13 43 , in An. funestus mosquitoes from Benin 45 and An. arabiensis from Ethiopia 44 .
Our current experiment showed that repeated exposure of laboratory colony of An. gambiae Tiassalé strain larvae to deltamethrin and adult to PermaNet 2.0 LLIN or combining exposure at both larvae and adult stages create pyrethroid resistance and select for P450 detoxification enzymes. The selection of metabolic resistance genes and increase in phenotype resistance in kdr resistance- An. gambiae, highlight the importance of metabolic resistance mechanism in the overall resistance phenomena. This suggests in our case that when selection pressure is high upon exposure to pyrethroids, mosquitoes with metabolic resistance background are the most selected. However, an important caveat is that we did not determine the intensity of pyrethroid resistance, which could be useful in measuring the strength of deltamethrin insecticide tested in this study. Wide use of insecticides ( e.g., deltamethrin) in agriculture for crop protection and pyrethroid-treated vector control tools in public health are seriously threatening the efficacy of existing vector control programmes by inducing and spreading metabolic resistance in local mosquito vectors. Therefore, there is a need to integrate new classes of insecticides (neonicotinoids, pyrroles) into malaria vector control strategies to delay the spread of pyrethroid resistance. Based on this study, it would be advisable for vector control strategies to extend beyond the search for kdr mutation only, as this does not appear to play more important role than metabolic resistance. Characterization of metabolic resistance should be an integrated part of insecticide resistance investigation prior to a vector control strategy implementation.
Conclusions
Repeated exposure of kdr resistant- An. gambiae Tiassalé strain as larvae to a sublethal dose of deltamethrin and adults to PermaNet 2.0 LLIN and combining exposure over 20 generations in laboratory demonstrated an induction of very high level of resistance to deltamethrin triggered by the cytochromes P450 detoxification enzymes. Additionally, during the experiments, increased occurrences of these genes was proportionally associated with significant reductions in susceptibility and mortality of exposed groups against deltamethrin. However, the kdr Vgsc-L995F frequency remained very high from parental generation to the end of the experiment. Prospective studies to implement control strategies must consider investigating metabolic resistance mechanisms in the target population and not solely kdr resistance mechanisms.
Acknowledgments
We thank all CSRS technicians for their technical assistance at the CSRS insectary. We are grateful to Emmanuel Mbuba from Ifakara Health Institute Institute and Zoh Marius from CSRS for providing relevant criticism on the first version of the manuscript.
Funding Statement
This work was supported by the Wellcome (103995).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved, 1 approved with reservations]
Data availability
Zenodo. Phenotypic resistance to pyrethroid associated to metabolic mechanism in Vgsc-L995F resistant-Anopheles gambiae malaria mosquitoes. DOI: https://doi.org/10.5281/zenodo.7669512 48
Data are available under the terms of Creative Commons Zero “No rights reserved” data waiver (CC-BY 4.0 Public domain dedication)
References
- 1. WHO: World malaria report.Geneva: World Health Organization; 2022. Reference Source
- 2. Ranson H, N'Guessan R, Lines J, et al. : Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27(2):91–8. 10.1016/j.pt.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 3. Ahoua Alou LP, Koffi AA, Adja MA, et al. : Status of pyrethroid resistance in Anopheles gambiae s. s. M form prior to the scaling up of Long Lasting Insecticidal Nets (LLINs) in Adzopé, Eastern Côte d'Ivoire. Parasit Vectors. 2012;5:289. 10.1186/1756-3305-5-289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Glunt KD, Coetzee M, Huijben S, et al. : Empirical and theoretical investigation into the potential impacts of insecticide resistance on the effectiveness of insecticide-treated bed nets. Evol Appl. 2018;11(4):431–41. 10.1111/eva.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fodjo BK, Koudou BG, Tia E, et al. : Insecticides Resistance Status of An. gambiae in Areas of Varying Agrochemical Use in Côte D'Ivoire. Biomed Res Int. 2018;2018: 2874160. 10.1155/2018/2874160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reid MC, McKenzie FE: The contribution of agricultural insecticide use to increasing insecticide resistance in African malaria vectors. Malar J. 2016;15:107. 10.1186/s12936-016-1162-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. WHO: Test procedures for insecticide resistance monitoring in malaria vector mosquitoes.Geneva: World Health Organization; 2016. Reference Source [Google Scholar]
- 8. Protopopoff N, Mosha JF, Lukole E, et al. : Effectiveness of a long-lasting piperonyl butoxide-treated insecticidal net and indoor residual spray interventions, separately and together, against malaria transmitted by pyrethroid-resistant mosquitoes: a cluster, randomised controlled, two-by-two factorial design trial. Lancet. 2018;391(10130):1577–88. 10.1016/S0140-6736(18)30427-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tiono AB, Ouédraogo A, Ouattara D, et al. : Efficacy of Olyset Duo, a bednet containing pyriproxyfen and permethrin, versus a permethrin-only net against clinical malaria in an area with highly pyrethroid-resistant vectors in rural Burkina Faso: a cluster-randomised controlled trial. Lancet. 2018;392(10147):569–80. 10.1016/S0140-6736(18)31711-2 [DOI] [PubMed] [Google Scholar]
- 10. WHO: Conditions for deployment of mosquito nets treated with a pyrethroid and piperonyl butoxide. Geneva: World Health Organization; 2017. Reference Source [Google Scholar]
- 11. Jones CM, Liyanapathirana M, Agossa FR, et al. : Footprints of positive selection associated with a mutation ( N1575Y) in the voltage-gated sodium channel of Anopheles gambiae. Proc Natl Acad Sci U S A. 2012;109(17):6614–9. 10.1073/pnas.1201475109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diabate A, Baldet T, Chandre F, et al. : The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67(6):617–22. 10.4269/ajtmh.2002.67.617 [DOI] [PubMed] [Google Scholar]
- 13. Camara S, Koffi AA, Ahoua Alou LP, et al. : Mapping insecticide resistance in Anopheles gambiae (s.l.) from Cote d'Ivoire. Parasit Vectors. 2018;11(1):19. 10.1186/s13071-017-2546-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hemingway J: The role of vector control in stopping the transmission of malaria: threats and opportunities. Philos Trans R Soc Lond B Biol Sci. 2014;369(1645): 20130431. 10.1098/rstb.2013.0431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ranson H, Claudianos C, Ortelli F, et al. : Evolution of supergene families associated with insecticide resistance. Science. 2002;298(5591):179–81. 10.1126/science.1076781 [DOI] [PubMed] [Google Scholar]
- 16. Li X, Schuler MA, Berenbaum MR: Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu Rev Entomol. 2007;52:231–53. 10.1146/annurev.ento.51.110104.151104 [DOI] [PubMed] [Google Scholar]
- 17. Ibrahim SS, Riveron JM, Stott R, et al. : The cytochrome P450 CYP6P4 is responsible for the high pyrethroid resistance in knockdown resistance-free Anopheles arabiensis. Insect Biochem Mol Biol. 2016;68:23–32. 10.1016/j.ibmb.2015.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Müller P, Warr E, Stevenson BJ, et al. : Field-caught permethrin-resistant Anopheles gambiae overexpress CYP6P3, a P450 that metabolises pyrethroids. PLoS Genet. 2008;4(11): e1000286. 10.1371/journal.pgen.1000286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu N: Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu Rev Entomol. 2015;60:537–59. 10.1146/annurev-ento-010814-020828 [DOI] [PubMed] [Google Scholar]
- 20. Yahouédo GA, Chandre F, Rossignol M, et al. : Contributions of cuticle permeability and enzyme detoxification to pyrethroid resistance in the major malaria vector Anopheles gambiae. Sci Rep. 2017;7(1): 11091. 10.1038/s41598-017-11357-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chouaïbou MS, Fodjo BK, Fokou G, et al. : Influence of the agrochemicals used for rice and vegetable cultivation on insecticide resistance in malaria vectors in southern Cote d'Ivoire. Malar J. 2016;15(1):426. 10.1186/s12936-016-1481-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yadouleton AW, Asidi A, Djouaka RF, et al. : Development of vegetable farming: a cause of the emergence of insecticide resistance in populations of Anopheles gambiae in urban areas of Benin. Malar J. 2009;8:103. 10.1186/1475-2875-8-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. IFAD: Rural Development Report 2016. Rome;2016. Reference Source
- 24. Haggblade S, Diarra A, Traoré A: Regulating agricultural intensification: Lessons from West Africa’s rapidly growing pesticide markets. Dev Policy Rev. 2021;40(1). 10.1111/dpr.12545 [DOI] [Google Scholar]
- 25. Edi CA, Koudou BG, Bellai L, et al. : Long-term trends in Anopheles gambiae insecticide resistance in Cote d'Ivoire. Parasit Vectors. 2014;7:500. 10.1186/s13071-014-0500-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wipf NC, Duchemin W, Kouadio FA, et al. : Multi-insecticide resistant malaria vectors in the field remain susceptible to malathion, despite the presence of Ace1 point mutations. PLoS Genet. 2022;18(2): e1009963. 10.1371/journal.pgen.1009963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fodjo BK, Kropf A, Gonse Zoh M, et al. : Impact of sublethal pyrethroid exposure on resistant Anopheles gambiae mosquitoes’ fitness [version 1; peer review: 2 approved with reservations]. Wellcome Open Res. 2021;6:204. 10.12688/wellcomeopenres.17074.1 [DOI] [Google Scholar]
- 28. Mouhamadou CS, de Souza SS, Fodjo BK, et al. : Evidence of insecticide resistance selection in wild Anopheles coluzzii mosquitoes due to agricultural pesticide use. Infect Dis Poverty. 2019;8(1):64. 10.1186/s40249-019-0572-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Edi CV, Djogbenou L, Jenkins AM, et al. : CYP6 P450 enzymes and ACE-1 duplication produce extreme and multiple insecticide resistance in the malaria mosquito Anopheles gambiae. PLoS Genet. 2014;10(3): e1004236. 10.1371/journal.pgen.1004236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bass C, Nikou D, Donnelly MJ, et al. : Detection of knockdown resistance ( kdr) mutations in Anopheles gambiae: a comparison of two new high-throughput assays with existing methods. Malar J. 2007;6:111. 10.1186/1475-2875-6-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mavridis K, Wipf N, Müller P, et al. : Detection and Monitoring of Insecticide Resistance Mutations in Anopheles gambiae: Individual vs Pooled Specimens. Genes (Basel). 2018;9(10):479. 10.3390/genes9100479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mavridis K, Wipf N, Medves S, et al. : Rapid multiplex gene expression assays for monitoring metabolic resistance in the major malaria vector Anopheles gambiae. Parasit Vectors. 2019;12(1):9. 10.1186/s13071-018-3253-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. R Core Team: R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.2020. Reference Source [Google Scholar]
- 35. RStudio Team: RStudio: Integrated Development Environment for R. RStudio, PBC, Boston, MA.2020. [Google Scholar]
- 36. Stalpers LJA, Kaplan EL: Edward L. Kaplan and the Kaplan-Meier Survival Curve. BSHM Bulletin: Journal of the British Society for the History of Mathematics. 2018;33(2):109–35. Reference Source [Google Scholar]
- 37. Poupardin R, Reynaud S, Strode C, et al. : Cross-induction of detoxification genes by environmental xenobiotics and insecticides in the mosquito Aedes aegypti: impact on larval tolerance to chemical insecticides. Insect Biochem Mol Biol. 2008;38(5):540–51. 10.1016/j.ibmb.2008.01.004 [DOI] [PubMed] [Google Scholar]
- 38. Meiwald A, Clark E, Kristan M, et al. : Association of Reduced Long-Lasting Insecticidal Net Efficacy and Pyrethroid Insecticide Resistance With Overexpression of CYP6P4, CYP6P3, and CYP6Z1 in Populations of Anopheles coluzzii From Southeast Cote d'Ivoire. J Infect Dis. 2022;225(8):1424–34. 10.1093/infdis/jiaa699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Machani MG, Ochomo E, Zhong D, et al. : Phenotypic, genotypic and biochemical changes during pyrethroid resistance selection in Anopheles gambiae mosquitoes. Sci Rep. 2020;10(1): 19063. 10.1038/s41598-020-75865-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shi L, Hu H, Ma K, et al. : Development of Resistance to Pyrethroid in Culex pipiens pallens Population under Different Insecticide Selection Pressures. PLoS Negl Trop Dis. 2015;9(8): e0003928. 10.1371/journal.pntd.0003928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Grossman MK, Uc-Puc V, Rodriguez J, et al. : Restoration of pyrethroid susceptibility in a highly resistant Aedes aegypti population. Biol Lett. 2018;14(6): 20180022. 10.1098/rsbl.2018.0022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yunta C, Hemmings K, Stevenson B, et al. : Cross-resistance profiles of malaria mosquito P450s associated with pyrethroid resistance against WHO insecticides. Pestic Biochem Physiol. 2019;161:61–7. 10.1016/j.pestbp.2019.06.007 [DOI] [PubMed] [Google Scholar]
- 43. Williams J, Flood L, Praulins G, et al. : Characterisation of Anopheles strains used for laboratory screening of new vector control products. Parasit Vectors. 2019;12(1):522. 10.1186/s13071-019-3774-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Messenger LA, Impoinvil LM, Derilus D, et al. : A whole transcriptomic approach provides novel insights into the molecular basis of organophosphate and pyrethroid resistance in Anopheles arabiensis from Ethiopia. Insect Biochem Mol Biol. 2021;139: 103655. 10.1016/j.ibmb.2021.103655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Riveron JM, Yunta C, Ibrahim SS, et al. : A single mutation in the GSTe2 gene allows tracking of metabolically based insecticide resistance in a major malaria vector. Genome Biol. 2014;15(2):R27. 10.1186/gb-2014-15-2-r27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Simma EA, Dermauw W, Balabanidou V, et al. : Genome-wide gene expression profiling reveals that cuticle alterations and P450 detoxification are associated with deltamethrin and DDT resistance in Anopheles arabiensis populations from Ethiopia. Pest Manag Sci. 2019;75(7):1808–1818. 10.1002/ps.5374 [DOI] [PubMed] [Google Scholar]
- 47. Balabanidou V, Kampouraki A, MacLean M, et al. : Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proc Natl Acad Sci U S A. 2016;113(33):9268–73. 10.1073/pnas.1608295113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kouadio France-Paraudie A: Phenotypic resistance to pyrethroid associated to metabolic mechanism in Vgsc-L995F resistant- Anopheles gambiae malaria mosquitoes. In: Zenodo, editor.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]