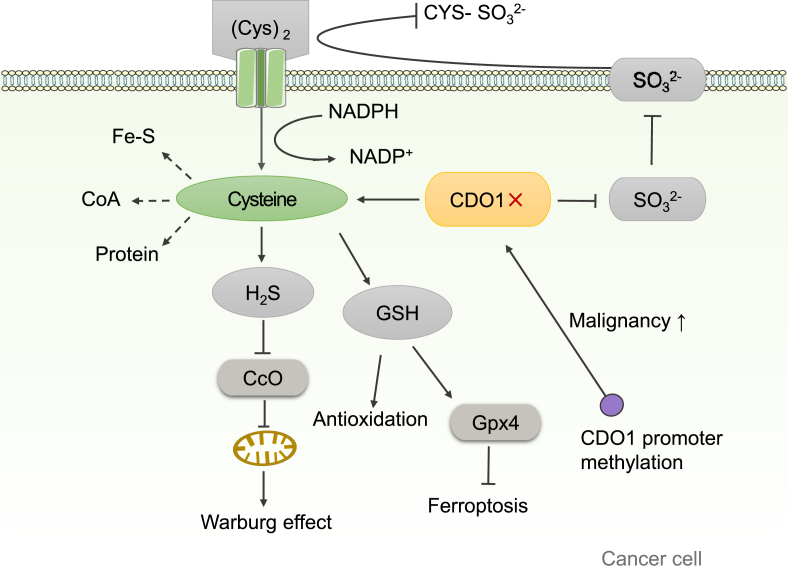
Figure 5.
The effect of CDO1 on cancer. CDO1 reduces intracellular level of cysteine by catabolizing cysteine. Down-regulation of CDO1 increases the intracellular level of cysteine by decreasing the catabolism of cysteine. In addition, CDO1 silencing can reduce the production of and inhibit the reaction of cystine with to produce cysteine-S-sulfate . Because cystine is the resource for intracellular cysteine, reduced formation of will increase the level of free cystine, thereby indirectly increasing intracellular cysteine level. Intracellular cysteine is involved in the synthesis of proteins, CoA and Fe-S, which are important for cancer cell proliferation. Furthermore, extracellular cystine can enter the cell through its transporter and NADPH as an electron donor for the production of cysteine from cystine, which consumes a large amount of NADPH. Silencing of CDO1 will increase the intracellular level of cysteine, which would decrease the need for the transportation of cystine from extracellular area, leading to decreased consumption of NADPH and increased NADPH/NADP+ ratio. It is known that increased NADPH/NADP + ratio will promote cancer cell proliferation. Increased promoter methylation of CDO1 results in down-regulation of CDO1 gene expression, thereby increasing the malignancy of cancer. In cancer cells, CDO1 silencing can promote the production of GSH, and then improve the adaption of cancer cells to oxidative stress. In addition, when CDO1 is inhibited, the synthesis of GSH increased, which in turn increases the activity of GPX4 and inhibits the ferroptosis of cancer cells. Furthermore, CDO1 silencing leads to the increase of H2S production, which inhibits mitochondrial function and in turn promotes Warburg effect in cancer cells. Therefore, CDO1 could function as a tumor suppressor gene. Fe-S, iron-sulfur clusters; CcO, cytochrome c oxidase; Gpx4, glutathione peroxidase 4.