Figure 5. The M5 mutant phenocopies loopless Ndc80 in vivo and in vitro .
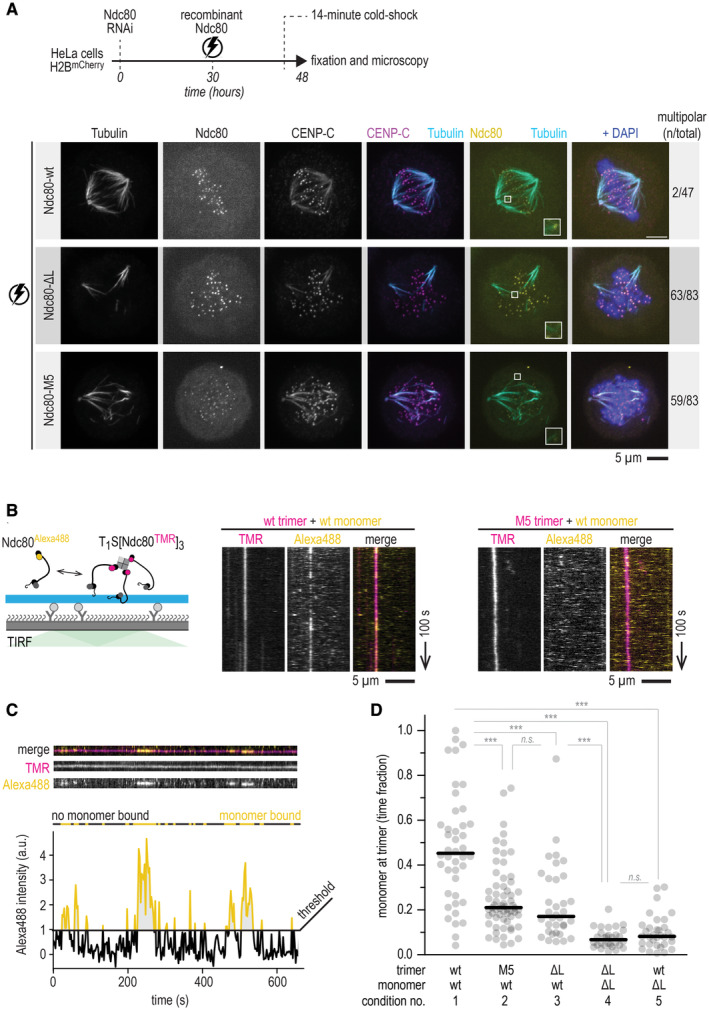
- Schematic of a cold‐shock assay following an electroporation experiment. Immunofluorescence images showing the attachment status of kinetochores to microtubules in cells electroporated with recombinant Ndc80‐wt, Ndc80‐∆L, or Ndc80‐M5 complexes. The number of cells with multipolar spindles and the total number of analyzed cells are shown. Some signal from the tubulin channel is visible in the CENP‐C channel. Scale bar: 5 μm.
- Total Internal Reflection Fluorescence (TIRF) microscopy was used to investigate Ndc80Alexa488 complexes (0.6 nM) added to trimeric Ndc80TMR (10 pM) on fluorescent taxol‐stabilized microtubules that were attached to a passivated glass surface. Typical kymographs showing virtually motionless Ndc80 trimers (magenta) and transiently binding Ndc80 monomers (yellow). Wild‐type (wt) monomers associate with wt trimers (left), but not with M5 trimers (right). Scale bars: vertical (100 s), horizontal (5 μm).
- Quantification of the intensity of the monomeric Ndc80 associating with microtubule‐bound trimeric Ndc80. A threshold for binding was set at an intensity equivalent to one Alexa488 copy. Intensities well above 1 (yellow) could thus reflects multiple monomers binding simultaneously.
- Fraction of time there was at least one monomer (added to solution at a concentration of 0.6 nM) present at the microtubule‐bound trimer (10 pM), tested in various combinations of wild‐type (wt), loopless (ΔL) and M5 monomers or trimers. All analyzed traces of Ndc80 trimers are shown (n = 42, 63, 33, 30, 34 for conditions 1–5). Horizontal lines show median values and statistical significance was determined using a two‐tailed Mann–Whitney test. P‐values: 1 (wt‐trimer + wt‐monomer) vs. 2 (M5‐trimer + wt‐monomer): 7·10−7 (***); 2 vs. 3: 0.17 (n.s.); 1 vs. 3: 1·10−6 (***); 1 vs. 4: 3·10−15 (***); 3 vs. 4: 1·10−8 (***); 1 vs. 5: 1·10−13 (***); 4 vs. 5 0.23 (n.s.).
Source data are available online for this figure.
