FIG 9.
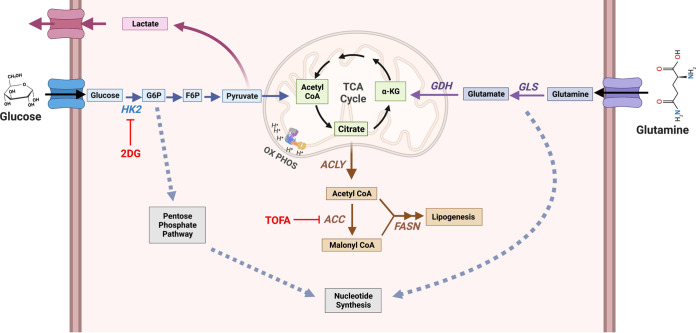
Model of host cell metabolism changes during lytic gammaherpesvirus infection. Our global metabolomics and lipidomics indicate that during MHV-68 lytic infection of host NIH 3T3 cells, metabolic intermediates found in glycolysis, glutaminolysis, lipogenesis, and nucleotide metabolism are significantly elevated over mock-infected controls. Briefly, glucose is taken up by the host cell via membrane-bound glucose transporter proteins and metabolized by hexokinase (HK) to form glucose-6-phosphate (G-6-P). Depending on cellular or viral need, G-6-P can have several fates, including (i) being further metabolized through glycolysis to form pyruvate to fuel the TCA cycle in the mitochondria, (ii) being converted from pyruvate to lactate and secreted outside the cell, or (iii) fueling the pentose phosphate pathway for nucleotide synthesis. During glutaminolysis, glutamine is taken up by the host cell via a membrane-bound glutamine transporter. Glutamine is converted to glutamate and NH3 by glutaminase (GLS) and then to α-ketoglutarate by glutamate dehydrogenase (GDH). Next, α-ketoglutarate enters and replenishes the TCA cycle. Glutamine breakdown can be used for nucleotide synthesis. Overall, downstream intermediates from both glucose and glutamine, which feed into the TCA cycle, can fuel the lipogenesis pathway. Briefly, citrate is converted to cytoplasmic acetyl-CoA by ATP citrate lyase (ACLY) and then malonyl-CoA by acetyl-CoA carboxylase (ACC). Acetyl-CoA and malonyl-CoA combine to form palmitic acid by fatty acid synthase (FASN), which is then used as a backbone to make longer-chain fatty acids, triglycerides, phospholipids, and other lipid materials. During MHV-68 lytic infection, the expression of key enzymes in these pathways, including GDH, ACLY, and FASN, are elevated. Figure created with BioRender.com.