FIG 3.
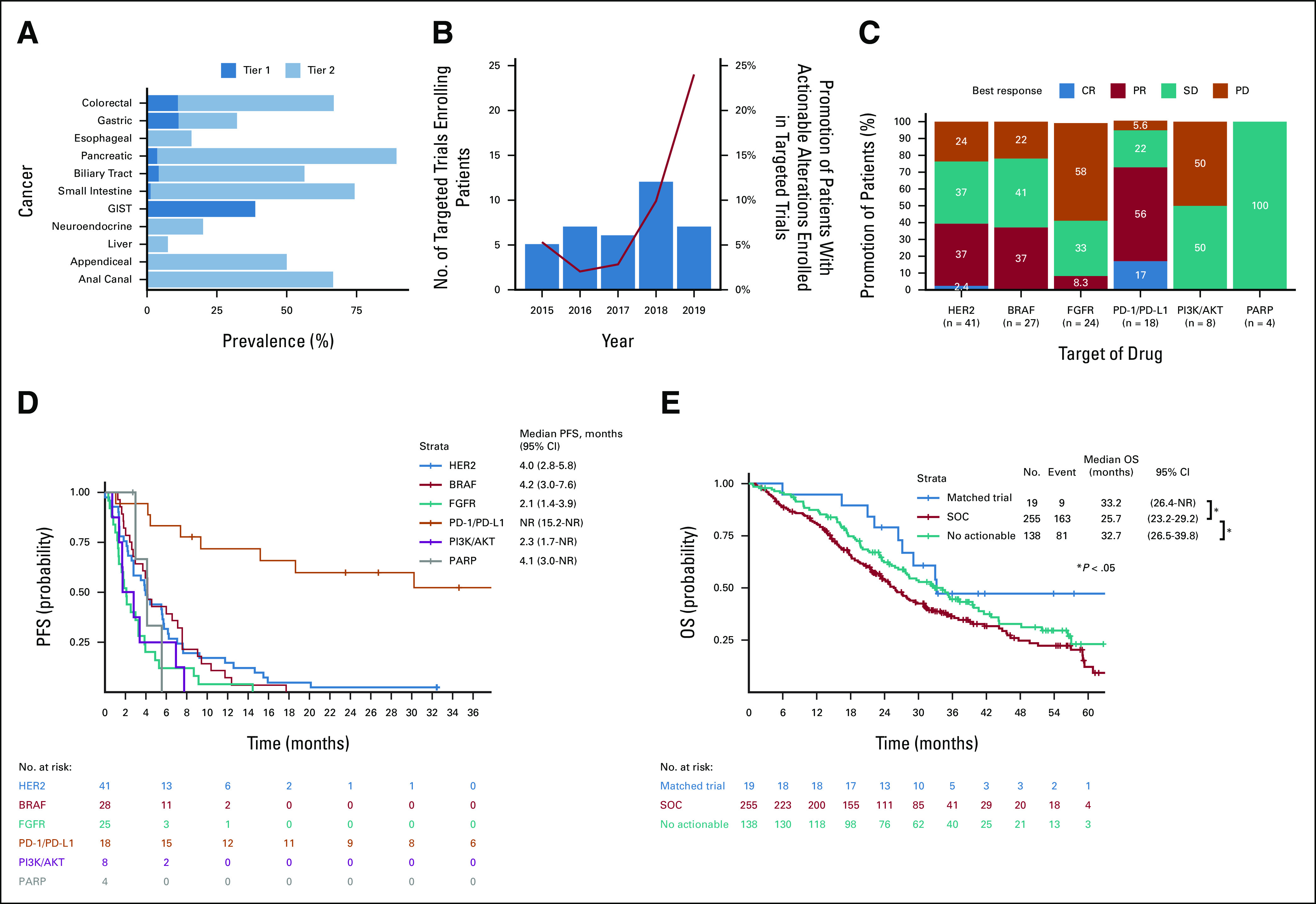
Efficacy of matched therapies in patients enrolled in affiliated clinical trials. (A) The prevalence of patients with tier 1 or 2 genomic alterations as the most actionable alterations. (B) Number of targeted trials enrolling patients in GI-SCREEN (box plot) and proportion of patients enrolled in a matched clinical trial among those with actionable alterations (line plot). (C) Number of patients who had tumor responses according to RECIST v1.1 by matched therapies in the matched clinical trials. (D) Kaplan-Meier curves of PFS of patients receiving matched therapies in matched clinical trials. (E) Kaplan-Meier curves of OS of treatment-naïve patients with metastatic colorectal cancer harboring actionable alterations receiving matched therapies in matched clinical trials and SOC and no actionable alterations. CR, complete response; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; SOC, standard of care.
