FIG 2.
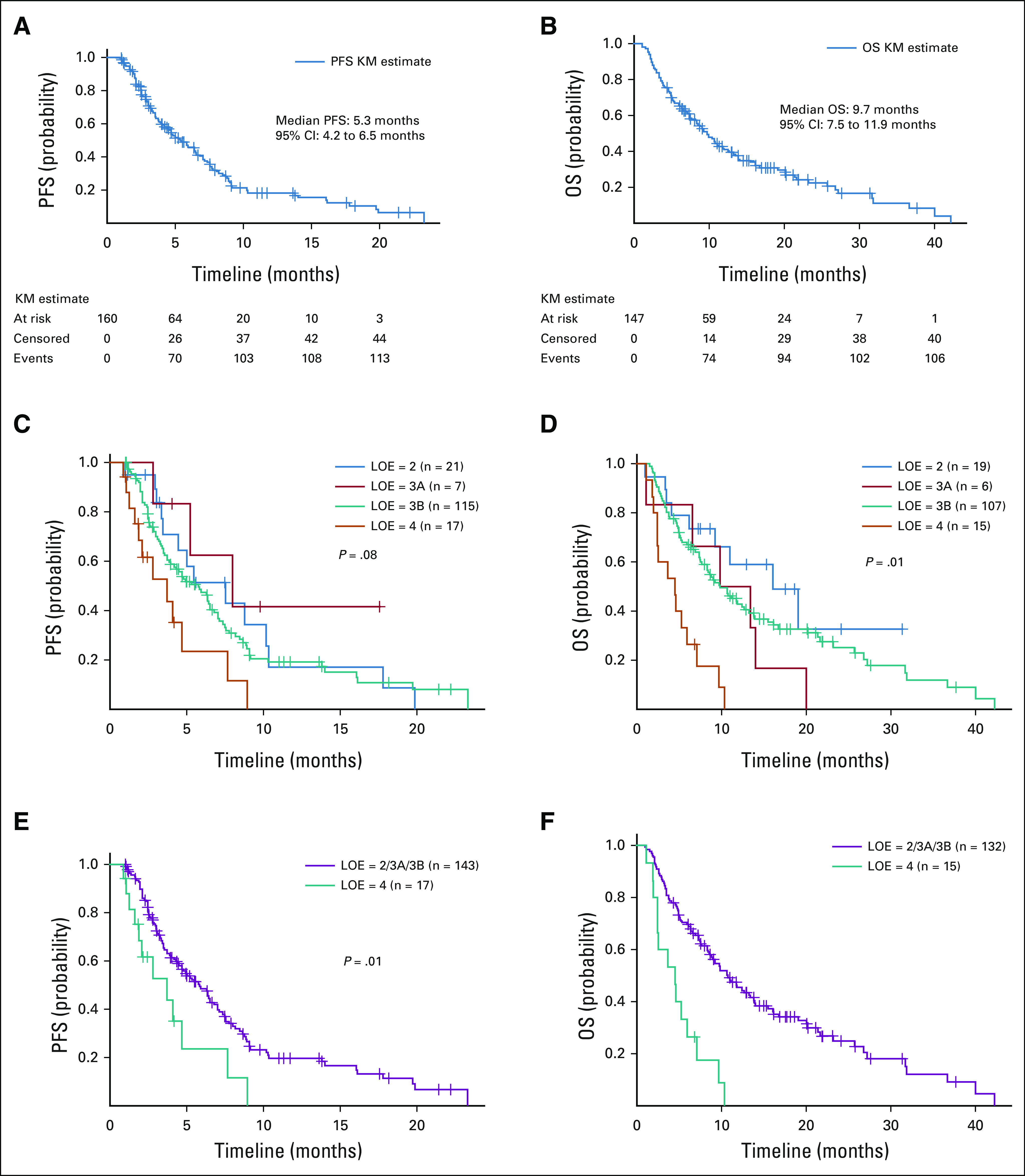
(A) PFS and (B) OS curves for all patients who were treated with off-label TAs for more than 4 weeks. (C) PFS and (D) OS curves based upon OncoKB therapeutic LOE. The median PFS was 7.5 months (95% CI, 3.5 to 10.3) for LOE = 2 group, 8.0 months (95% CI, 2.8 to NR) for LOE = 3A group, 5.8 months (95% CI, 3.9 to 6.5) for LOE = 3B group, and 3.7 months (95% CI, 1.6 to 7.7) for LOE = 4 group. The median OS was 16.1 months (95% CI, 6.2 to NR) for LOE = 2 group, 13.4 months (95% CI, 1.1 to 20.1) for LOE = 3A group, 9.9 months (95% CI, 7.5 to 13.2) for LOE = 3B group, and 4.5 months (95% CI, 2.0 to 5.9) for LOE = 4 group. (E) PFS and (F) OS curves for LOE two groups: 2/3A/3B versus 4. The median PFS was 5.8 months (95% CI, 4.5 to 7.0) for LOE = 2/3A/3B group and 3.7 months (95% CI, 1.6 to 7.7) for LOE = 4 group. The HR was 2.24 (95% CI, 1.22 to 4.11; P = .01) for being in group 4 compared with in group 2/3A/3B. The HR was 0.45 (95% CI, 0.24 to 0.82; P = .01) for being in group 2/3A/3B compared with in group 4. The median OS was 10.7 months (95% CI, 8.4 to 13.8) for LOE = 2/3A/3B group and 4.5 months (95% CI, 2.0 to 5.9) for LOE = 4 group. HR, hazard ratio; LOE, level of evidence; NR, not reached; OS, overall survival; PFS, progression-free survival; TA, targeted agent.
