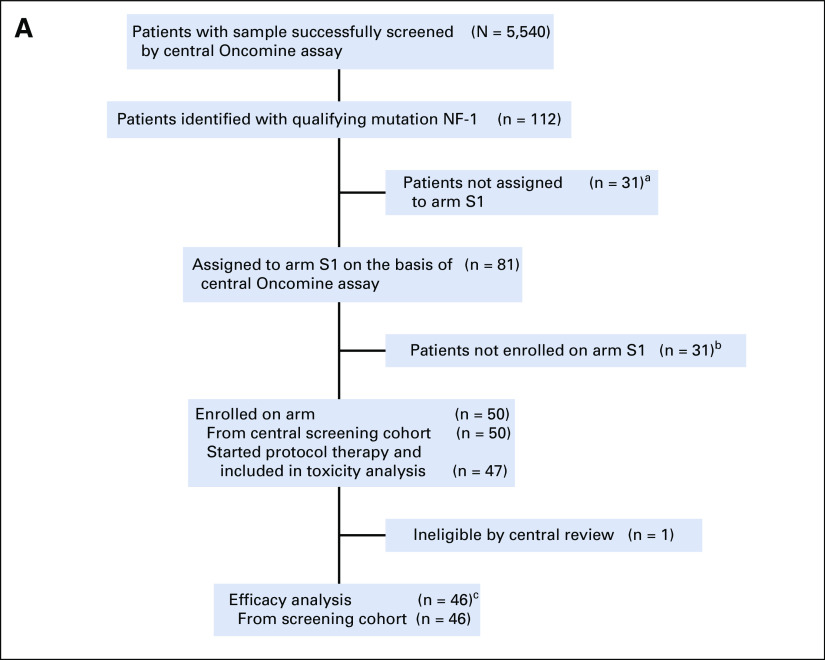
FIG 1.
Diagrams for subprotocols (A) S1 and (B) S2. aReasons for not receiving an assignment: study suspended (n = 15), assigned to another arm for which the qualifying variant’s level of evidence was higher (n = 7), before study activation (n = 6), assigned to another arm for which accrual was lower (n = 2), and prior treatment of trametinib (n = 1). bReasons not enrolled after receiving an assignment: active infection and abnormal brain MRI (n = 1), deteriorating performance status (n = 6), eye problems (n = 1), in hospice care (n = 1), inadequate organ/marrow function (n = 2), interstitial lung disease/pneumonitis (n = 2), intervening news/targeted treatment (n = 2), other investigational treatment (n = 1), patient died (n = 6), patient refusal (n = 4), receiving other therapy (n = 2), and unknown (n = 3). cReasons for being nonevaluable: no disease assessment done before death (n = 4), no disease assessment done before NPT (n = 1), baseline scans done outside of specified window (n = 5), withdrew consent (n = 2), different methods of evaluation used (n = 1), no evaluation during treatment (n = 1). dReasons for not receiving an assignment: ineligible histology—uveal melanoma (n = 13). eReasons not enrolled after receiving an assignment: central screening cohort: ineligible histology (n = 1), ineligible histology and inadequate organ/marrow function (n = 1), and unknown (n = 1). NPT, nonprotocol therapy.