Abstract
We retrospectively reviewed consecutive cases of mucormycosis reported from a tertiary-care center in India to determine the clinical and mycologic characteristics of emerging Rhizopus homothallicus fungus. The objectives were ascertaining the proportion of R. homothallicus infection and the 30-day mortality rate in rhino-orbital mucormycosis attributable to R. homothallicus compared with R. arrhizus. R. homothallicus accounted for 43 (6.8%) of the 631 cases of mucormycosis. R. homothallicus infection was independently associated with better survival (odds ratio [OR] 0.08 [95% CI 0.02–0.36]; p = 0.001) than for R. arrhizus infection (4/41 [9.8%] vs. 104/266 [39.1%]) after adjusting for age, intracranial involvement, and surgery. We also performed antifungal-susceptibility testing, which indicated a low range of MICs for R. homothallicus against the commonly used antifungals (amphotericin B [0.03–16], itraconazole [0.03–16], posaconazole [0.03–8], and isavuconazole [0.03–16]). 18S gene sequencing and amplified length polymorphism analysis revealed distinct clustering of R. homothallicus.
Keywords: Rhizopus homothallicus, Rhizopus arrhizus, mucormycosis, fungi, fungal infections, COVID-19 associated mucormycosis, epidemiology, antifungal susceptibility testing, amplified fragment length polymorphism, India
Mucormycosis is an angioinvasive disease caused by the saprophytic fungi of the order Mucorales. The estimated prevalence of mucormycosis is ≈70 times higher in India than elsewhere (1). Rhizopus arrhizus is the most common etiologic agent of mucormycosis in India and worldwide (2). Other reported Mucorales include Apophysomyces variabilis, Cunnighamella spp., Lichtheimia spp., Mucor spp., Rhizomucor spp., Rhizopus microsporus, Rhizopus homothallicus, Saksenaea vasiformis, Syncephalastrum spp., and Thamostylum lucnowense (3–7). R. arrhizus was the most common causative agent even during the recent outbreak of COVID-19–associated mucormycosis (CAM) in India (8). Although infection with R. homothallicus was also reported in a few patients (9), the importance of mucormycosis caused by R. homothallicus is unclear. We report the percentage of patients with mucormycosis caused by R. homothallicus at our center (Postgraduate Institute of Medical Education and Research [PGIMER], Chandigarh, India) and describe clinical features, mycologic characteristics, antifungal susceptibility, treatment, and mortality rates. We also assess whether the mortality rate from rhino-orbital mucormycosis (ROM) caused by R. homothallicus is different from that of R. arrhizus disease.
The primary objectives of this study were to assess the proportion of patients with mucormycosis caused by R. homothallicus and 30-day mortality rate from ROM caused by R. homothallicus and to determine whether the species of Mucorales (R. homothallicus vs. R. arrhizus) was an independent predictor of death from ROM. The secondary objectives were to compare the profile of patients infected with R. homothallicus versus R. arrhizus and to ascertain the mycologic characteristics of R. homothallicus isolates by conducting antifungal-susceptibility testing (AFST) and amplified fragment length polymorphism (AFLP) analysis.
Methods
Study Design and Setting
We performed a retrospective study at PGIMER on a 10-month period (January–October 2021). Our center’s institutional ethics committee approved the study protocol. We were granted a consent waiver because the study was a retrospective analysis of anonymized patient data. We report the study according to the Strengthening the Reporting of Observational Studies in Epidemiology statement (10). Data for a few participants published in this study have been reported in previous studies (11–13). We conducted the study in accordance with Declaration of Helsinki guidelines; the study was approved by the PGIMER Institutional Review Board (approval no. PGI/IEC/2021/001101) in August 2021.
Study Participants
We enrolled consecutive patients with ROM caused by R. homothallicus and R. arrhizus. The patients in whom mucormycosis caused by R. homothallicus and R. arrhizus was diagnosed were identified from our mycology laboratory records; we collected relevant clinical data from the patient records. We followed the study participants until discharge or 30 days after their mucormycosis was diagnosed. We sought any missing information for the study by contacting patients by telephone. We excluded patients for whom information was not adequate or not available. We obtained informed consent from all patients involved in the study.
Data Collection and Variables
For eligible patients with ROM, we retrieved data on age, sex, and risk factors for mucormycosis (e.g., diabetes mellitus, COVID-19 infection, organ transplantation, immunosuppressive therapy, hematologic malignancies). We defined diabetes mellitus as recently diagnosed if the disease was detected (hyperglycemia and glycated hemoglobin >6.5%) during the current illness. We also retrieved clinical details, including signs and symptoms of ROM, treatment, and outcome of all patients (noted on follow up at 30 days after mucormycosis diagnosis, irrespective of discharge from hospital). All the study participants received the standard of care treatment in accordance with our institutional protocol.
Study Definitions
Mucormycosis was diagnosed in patients with compatible clinical and radiologic features and confirmed by histopathologic or microbiologic methods, as previously described (8). We arbitrarily defined COVID-19–associated mucormycosis as mucormycosis diagnosed simultaneously with or within 3 months of virologically confirmed COVID-19 (14).
Phenotypic Identification of the Isolates
We inoculated tissue samples collected from patients on Sabouraud dextrose agar and dichloran rose bengal chloramphenicol (both from HiMedia, India) with benomyl. We isolated the Mucorales from the culture and confirmed them by demonstrating broad aseptate ribbon-shaped hyphae on direct microscopic examination of the samples. We performed phenotypic identification on the basis of the colony morphology (e.g., texture, growth rate, and color) and microscopic findings (i.e., lactophenol cotton blue mount prepared from slide culture) (15). We then summarized the distinctive features of R. arrhizus and R. homothallicus (Table 1; Figure 1).
Table 1. Mycologic characteristics of Rhizopus arrhizus and R. homothallicus based on macroscopic and microscopic findings of tissue samples from patients enrolled in a 10-month retrospective study, Chandigarh, India, January–October 2021*.
| Characteristic | R. homothallicus | R. arrhizus |
|---|---|---|
| Macroscopic appearance | ||
| Growth rate | Fast-growing colonies and sporulation relatively slower than R. arrhizus | Fast-growing colonies and sporulation relatively faster than R. homothallicus |
| Obverse surface growth |
Cottony, white colonies that turn grayish to olive brown with a variegated appearance; because of the large-sized zygospores, brownish tufts can be seen unevenly distributed throughout the mycelial growth |
Cottony, white colonies that turn gray with typical salt and pepper appearance |
| Reverse macroscopic findings |
Reverse surface has no pigment |
Reverse surface has no pigment |
| Microscopic findings | ||
| Hyphae | Aseptate hyphae, less prominent rhizoids; smooth and thick-walled intercalary chlamydospore | Aseptate hyphae, well-developed nodal rhizoids with occasional intercalary chlamydospores |
| Sporangiophore | Erect, unbranched, or dichotomously branched (length 300 – 2000 µm, width 5–30 µm) | Single or tufts of mostly unbranched sporangiophores (length 1000–2000 µm, width 7–18 µm) |
| Sporangium | Sparsely noted in cultures; when present, appears spherical and greyish brown (20– 140 µm) with conspicuous dark apophysis and subspherical columella | Spherical sporangia (20–250 µm) with short apophysis and spherical columella occupying 50% of sporangium |
| Sporangiospores | Spherical to broadly ellipsoidal (3–5 × 4–8 µm), hyaline, and thick-walled with less prominent striations | Lemon-shaped or subspherical to ellipsoidal (6–8 × 4.5–5 µm) with striations, rough surface |
| Zygospores | Homothallic†; abundant golden brown zygospores (60–100 µm) with stellate spinous projections, attached to large globose suspensor cells that are unequal in size | Heterothallic zygospores not seen on primary culture; red-brown spherical or laterally flattened (60–140 µm) with flat projections; suspensors are unequal, spherical to conical |
*Key features highlighted in bold. †Differentiation from other homothallic Rhizopus species that produce abundant zygospores (e.g., R. sexualis and R. azygosporus) is done based on size of the suspensor cells of the zygospore and by molecular identification using the internal transcribed spacer and 28S sequences of rDNA.
Figure 1.
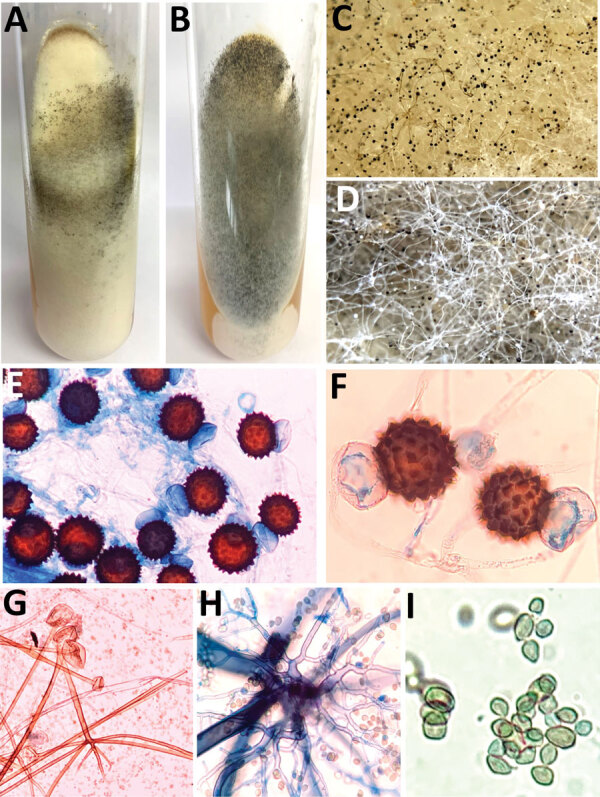
Macroscopic and microscopic characteristics of Rhizopus arrhizus and R. homothallicus isolated from tissue samples from patients enrolled in a 10-month retrospective study, Chandigarh, India, January–October 2021. A, B) Macroscopic appearance of colonies of R. homothallicus (A) and R. arrhizus (B) fungi. C, D) Macro lens image of the colonies showing dark-brown specks in R. homothallicus (C) and black and white dots (salt and pepper appearance) in R. arrhizus (D). E) Photomicrograph from a lactophenol cotton blue mount of R. homothallicus showing multiple reddish brown ornamented zygospores (original magnification ×400). F) Magnified image of E showing unequal suspenser cells and zygospore with prominent spinous projections. G) R. arrhizus showing long, unbranched sporangiophore with nodal rhizoids (original magnification ×200). H) Magnified image of extensively branched rhizoid seen in R. arrhizus. I) Magnified image of sporangiospores of R. arrhizus.
Molecular Identification of the Isolates
We confirmed identification of all R. homothallicus isolates from ROM cases and 6 additional R. homothallicus isolates from pulmonary mucormycosis cases by using a molecular sequencing method. In addition, we used sequences of 2 environmental isolates of R. homothallicus for comparison with clinical strains. We submitted to GenBank and included for phylogenetic analysis only isolates with good-quality sequences (Appendix Table 1). We included the DNA sequences of 1 isolate each of R. microsporus and R. arrhizus from our culture collection as controls. For DNA extraction, we used freshly grown sporulated culture suspended in 500 µL of lysis buffer and incubated for 5 minutes at 56°C. We then extracted DNA by using the phenol–chloroform–isoamyl alcohol (25:24:1) method, as previously described (16). We used PCR-based amplification of the 18S, internal transcribed spacer, and 28S genes, followed by sequencing using previously published primers for molecular identification (17). Because we obtained mixed chromatograms in most isolates of R. homothallicus by sequencing the internal transcribed spacer and 28S regions, we used the sequences of 18S for phylogenetic analysis. We aligned all sequences of the study isolates and reference sequences of various Rhizopus species obtained from GenBank by using MEGA 7.0 (18). We studied the evolutionary relationship of isolates by constructing a phylogenetic tree by using neighbor-joining analysis in MEGA 7.0.
AFST
We performed AFST of the isolates for amphotericin B, itraconazole, posaconazole, isavuconazole, and terbinafine in accordance with Clinical and Laboratory Standards Institute (CLSI) standards (19). In brief, we diluted the drugs in the standard Roswell Park Memorial Institute 1640 medium and then dispensed 100 μL into each well of 96-well microdilution trays. We tested MICs in the range of 0.03–16 μg/mL. We prepared the inoculum by harvesting the zygospores in 0.85% normal saline and adjusted spore counts by using a spectrophotometer at an absorbance of 530 nm. On adjusting the optical density (OD) to limits of 0.15–0.17 (0.4–5.0 × 104 zygospores/mL), we observed no growth on subsequent incubation; hence, we used a higher OD of 0.2–0.3 (0.3–5.0 × 106 zygospores/mL, confirmed by counting on hemocytometer). We diluted the suspensions to 1:50 in Roswell Park Memorial Institute 1640 medium and incubated the microtiter plates at 35°C for 4–8 days until visible growth was observed in the growth control. We defined MIC endpoints as the lowest drug concentration that inhibited any recognizable growth. We used Candida krusei (ATCC 6258) and Aspergillus flavus (ATCC 204304) as the quality-control strains.
Because of the absence or presence of fewer sporangiospores in R. homothallicus, performing AFST was challenging, and we modified the standard CLSI guidelines of AFST for this study. We used a higher OD with more inoculum containing both zygospores and hyphal fragments; performed intermittent vortexing of the inoculum for up to 45 minutes to ensure homogenous suspension (because zygospores tend to settle fast and are sometimes unevenly distributed between the wells); and conducted reading of AFST results up to 7 days (because zygospores take longer time to germinate or multiply compared with sporangiospores). The last step is in contrast to the standard protocol of reading the results at 24 hours for other medically important Mucorales, according to CLSI (19).
AFLP Analysis
We performed AFLP typing of R. homothallicus isolates as previously described (20). We used ≈50 ng of genomic DNA for the combined restriction–ligation procedure. We fragmented the DNA by using the 5 units each of restriction enzymes EcoRI and HindIII (New England Biolabs). For the preselective amplification, we used 10 μmol/L each of preselective primers of EcoRI primer (5′-GACTGCGTACCAATTC-3′) and HindIII (5′-GACTGCGTACCA GCTT-3′). We used the 6-carboxyfluorescein (6-FAM) labeled primers for selective amplification. We used 10 μmol/L each of HindIII primer (5′-ACTGCGTACCAGCTTT-3′) and EcoRI primer (5′-GACTGCGTACCAATTCAC-3′) for selective amplification. We performed capillary electrophoresis to identify the restricted DNA fragments and reference marker (LIZ 500) in a genetic analyzer (3500 Genetic Analyzer 8 Capillary Array; Applied Biosystems). We imported the fingerprint data into BioNumerics 6.6 (Applied Maths) and converted curves into bands for analysis. We calculated the genetic diversity by using the Pearson correlation coefficient and clustered the isolates by using the unweighted pair group method with arithmetic mean. Although no definite cutoff for differentiating between strains exists, we used an arbitrary cutoff of <70% for genus differentiation and >70% for species differentiation. We defined clonal isolates when the similarity was in the range of 95%–99%.
Statistical Analysis
For statistical analysis, we used SPSS Statistics 22.0 (IBM) and GraphPad Prism 9.0 (GraphPad Software). We present the descriptive data as mean +SD for continuous variables and frequencies with percentages for categorical variables. We compared differences between the 2 groups by using the χ2 test and Fisher exact test for categorical variables, as appropriate, and used the Student t-test test to compare continuous data. We performed a binary logistic regression analysis of variables (age, sex, presence of brain involvement, combined medical–surgical therapy for ROM, and causative species [R. homothallicus vs. R. arrhizus]) that influenced the mortality rate for ROM and reported the adjusted odds ratio (aOR) and 95% CI. All the statistical tests were 2-sided, and we considered a p value <0.05 to be statistically significant.
Results
Of the 631 patients with culture-confirmed mucormycosis, 43 (6.8%) had infection attributable to R. homothallicus. We excluded 324 cases from the study (262 because of inadequate information [e.g., lost to follow up], 35 because of non–R. arrhizus and non–R. homothallicus mucormycosis, and 27 non-ROM cases). We analyzed 41 ROM cases caused by R. homothallicus and 266 consecutive ROM cases attributable to R. arrhizus.
The mean +SD age of the 307 patients with ROM was 51.6 years +12.8 years, and most were men (205 [66.8%]). The mean age of patients with R. arrhizus infection was significantly higher than that for patients with ROM caused by R. homothallicus. Data on diabetes status were available for 279 patients; 93.9% had diabetes, and of those, 45 (16.1%) had diabetic ketoacidosis during the initial encounter. We observed no statistically significant difference between the risk factors proportion of patients with intracranial involvement or medical and surgical management in the 2 study groups (Table 2). The overall mortality rate was 35.2% (108/307) and was significantly higher among patients infected with R. arrhizus compared with R. homothallicus (39.1% vs. 9.8%; p = 0.0001). The mortality rate of patients with ROM caused by R. homothallicus was not significantly different between CAM and non-CAM subgroups (Appendix Table 2).
Table 2. Comparison of mucormycosis caused by Rhizopus homothallicus versus R. arrhizus in patients enrolled in a 10-month retrospective study, Chandigarh, India, January–October 2021*.
| Parameter | R. homothallicus, n = 41 | R. arrhizus, n = 266 | p value |
|---|---|---|---|
| Mean age, y (+ SD) |
45.9 (± 12.8) |
52.5 (± 12.7) |
0.002 |
| Sex | |||
| M | 23/41 (56.1) | 182/266 (68.4) | 0.15 |
| F |
18/41 (43.9) |
84/266 (31.6) |
|
| Risk factors | |||
| CAM | 23/41 (56.1) | 256/266 (96.2) | 0.0001 |
| Duration after COVID-19, d (+ SD) | 6.13 (± 13.5) | 11.9 (± 14.8) | 0.07 |
| Diabetes mellitus | 39/41 (95.1) | 223/238 (93.7) | 0.72 |
| Recently diagnosed diabetes mellitus | 9/39 (23.1) | 54/223 (24.2) | |
| Renal transplantation |
0 |
1/238 (0.4) |
1.00 |
| Intracranial involvement |
3/41 (7.3) |
13/266 (4.9) |
0.46 |
| Clinical features | |||
| Fever | 4/41 (9.8) | 3/55 (5.5) | 0.42 |
| Headache | 4/41 (9.8) | 10/55 (18.2) | 0.38 |
| Toothache | 4/41 (9.8) | 5/55 (9.1) | 0.91 |
| Eye swelling | 27/41 (65.9) | 27/55 (49.1) | 0.10 |
| Facial pain | 11/41 (26.8) | 22/55 (40) | 0.18 |
| Facial swelling | 16/41 (39) | 22/55 (40) | 0.92 |
| Proptosis | 3/41 (7.3) | 0 | 0.08 |
| Visual disturbance | 18/41 (43.9) | 7/55 (12.7) | 0.0005 |
| Oral ulcer | 3/41 (7.3) | 10/55 (18.2) | 0.12 |
| Nasal crust | 5/41 (12.2) | 5/55 (9.1) | 0.62 |
| Palatal eschar |
5/41 (12.2) |
10/55 (18.2) |
0.42 |
| Management | |||
| Amphotericin therapy | 38/41 (92.7) | 212/218 (97.2) | 0.14 |
| LAMB | 36/38 (94.7) | 196/212 (92.5) | |
| Conventional AMB | 2/38 (5.3) | 16/212 (7.5) | |
| Surgery |
24/36 (66.7) |
184/245 (75.1) |
0.31 |
| 30-day mortality | 4/41 (9.8) | 104/266 (39.1) | 0.0001 |
*Values are no. patients/no. with data available (%) except as indicated. CAM, COVID-19–associated mucormycosis; LAMB, liposomal amphotericin B; AMB, amphotericin.
On multivariate analysis, infection with R. homothallicus and surgery for ROM were independently associated with lower odds of death. A higher age (odds ratio [OR] 1.01 [95% CI 1.03–1.08]) and the presence of brain involvement (OR 22.7 [95% CI 4.03–128.10]) were independently associated with higher mortality rates among ROM cases (Table 3).
Table 3. Binary logistic regression analysis demonstrating the factors associated with death among patients with rhino-orbital mucormycosis enrolled in a 10-month retrospective study, Chandigarh, India, January–October 2021*.
| Variable | Survivors | Nonsurvivors | Odds ratio (95% CI) | p value |
|---|---|---|---|---|
| Mean age, y (+ SD) | 55.8 (+ 12.4) | 49.4 (+ 12.6) | 1.06 (1.03–1.08) | 0.0001 |
| Male sex | 124/199 (62.3) | 81/108 (75) | 1.38 (0.75–2.53) | 0.31 |
| Intracranial involvement | 4/199 (2) | 12/108 (11.1) | 22.7 (4.03–128.1) | 0.0001 |
| Surgery for ROM | 144/173 (83.2) | 64/108 (59.3) | 0.22 (0.11–0.43) | 0.0001 |
| R. homothallicus infection | 37/199 (18.6) | 4/108 (3.7) | 0.08 (0.02–0.36) | 0.001 |
*Values are no. patients/no. with data available (%) except as indicated. ROM, rhino-orbital mucormycosis.
We performed AFST for 34 R. homothallicus isolates. We calculated the geometric mean, range, and MICs at which 50% (MIC50) and 90% (MIC90) of isolates are inhibited for amphotericin B, itraconazole, posaconazole, isavuconazole, and terbinafine (Table 4).
Table 4. Distribution of MICs of 34 Rhizopus homothallicus isolates from patients enrolled in a 10-month retrospective study, Chandigarh, India, January–October 2021*.
| Antifungal agent | Geometric mean (range), mg/L | MIC50, mg/L | MIC90, mg/L |
|---|---|---|---|
| Amphotericin B | 0.75 (0.03–16) | 2 | 4 |
| Itraconazole | 0.51 (0.03–16) | 0.5 | 8 |
| Posaconazole | 0.24 (0.03–8) | 0.12 | 2 |
| Isavuconazole | 0.32 (0.03–16) | 0.25 | 2 |
| Terbinafine | 0.34 (0.03–16) | 0.25 | 4 |
*MIC50, MIC at which 50% of isolates are inhibited; MIC90, MIC at which 90% of isolates are inhibited.
The phylogram constructed using 18S sequences (40 clinical and 2 environmental of R. homothallicus and 1 each of R. arrhizus and R. microsporus) (Figure 2; Appendix Table 1) and the AFLP results (Appendix Figure) both revealed distinct clustering of R. homothallicus from the other tested species. We observed no prominent clades among isolates of R. homothallicus from patients with CAM versus non-CAM.
Figure 2.
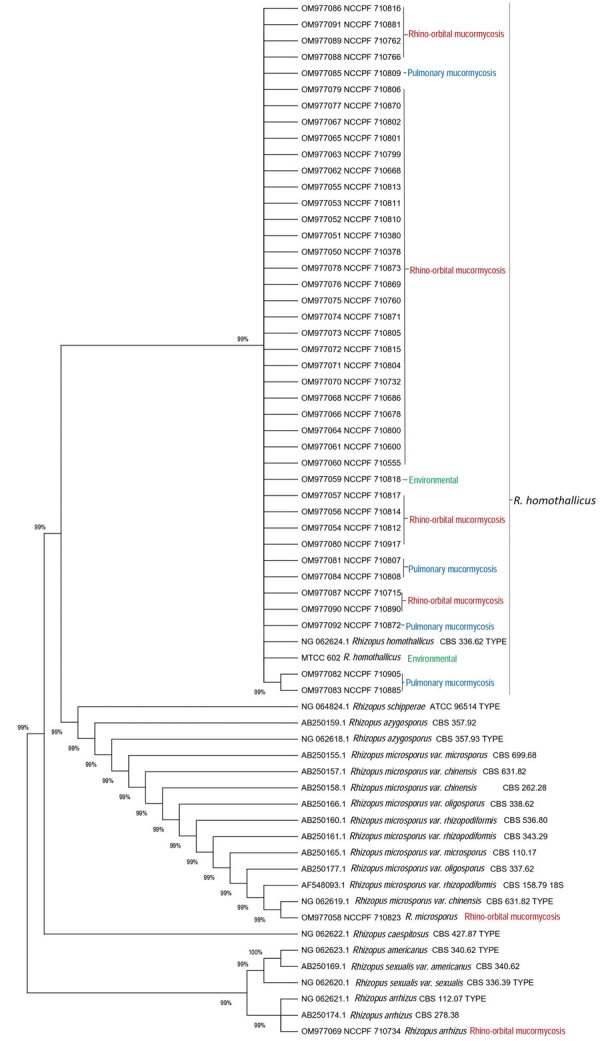
Evolutionary relationships of 40 Rhizopus homothallicus isolates from patients in a 10-month study, Chandigarh, India, January–October 2021, and 2 environmental isolates. GenBank accession numbers of 41 clinical and 1 environmental–MTCC 602 isolate of R. homothallicus from India are shown. Tree generated using neighbor-joining algorithm with 1,000 bootstrap replicates.
Discussion
We report a 6.8% prevalence of R. homothallicus infection among culture-confirmed mucormycosis cases. The mortality rate for ROM caused by R. homothallicus was significantly less than that for R. arrhizus (9.8% vs. 39.1%) after adjusting for age, sex, intracranial extension of the disease, and surgery for mucormycosis in the binary logistic regression model. The AFST data indicated good susceptibility to the common antifungal drugs and the newer agent isavuconazole. The 18S gene sequencing and AFLP revealed distinct clustering of R. homothallicus from the common species implicated in ROM.
Human infection with R. homothallicus was first reported from North India (4) in a case of pulmonary mucormycosis and was followed by a few more reports (5,21). In 2 prospective multicenter studies from India, R. homothallicus accounted for 2.5% (6/239) (22) and 7.6% (22/290) (23) of the cases where the causative organism could be identified. Despite a higher prevalance of mucormycosis cases (associated with the CAM outbreak) in our study, the prevalence of R. homothallicus mucormycosis (6.8%) was similar that in the previous reports.
R. homothallicus, a homothallic fungus, produces more heavy sexual spores than asexual spores (sporangiospores) and undergoes less dispersion in the air. The relative amount of sporangiospores is considerably lower in R. homothallicus than in the heterothallic Rhizopus species (24). Consequently, the chances of acquiring infection with R. homothallicus are expected to be low. Thus, R. homothallicus infections probably indicate the presence of some unidentified environmental niche where this agent can produce asexual spores abundantly and disperse them in the air. Recent studies have shown R. homothallicus in the hospital air and air samples obtained from the residence of patients with mucormycosis (25,26).
The mortality rate for mucormycosis may vary with the causative species. For instance, 2 systematic reviews have shown higher mortality rates for infections with Cunninghamella spp. than those with R. arrhizus (2,27). We observed a significantly better survival rate with R. homothallicus infection compared with R. arrhizus. A substantially higher proportion of patients with R. homothallicus infection had visual disturbance (44% vs. 13% for R. arrhizus), and this difference could have led to earlier detection. The duration of symptoms in CAM patients with mucormycosis caused by R. homothallicus and R. arrhizus was also different, although not significantly (6 vs. 12 days; p = 0.07). The timely initiation of antifungal therapy, intracranial spread of disease, and surgery for mucormycosis are important factors determining the outcome of mucormycosis (28,29). We conducted a preliminary growth curve analysis at 25°C, 37°C, and 40°C (data not shown) to determine whether the 2 species had a difference in growth rate. We observed an overall faster time to log phase at all temperatures for R. arrhizus compared with R. homothallicus (6 h vs. 27 h at 25°C, 5 h vs. 32 h at 37°C, and 6 h vs. 25 h at 40°C). However, further in vivo studies are required to identify the different pathogenic potentials of these 2 Mucorales species.
The first limitation of our study was that it was retrospective and conducted at a single center, limiting generalizability. We do not have information on all patients with ROM caused by R. arrhizus, and the proportion of patients with CAM was much higher in the R. arrhizus group than in the R homothallicus group. However, data from our center and another large multicenter study from India showed similar mortality rates for ROM with or without COVID-19 co-infection (8,11). Because our study focused exclusively on ROM cases, future studies should explore pulmonary or other sites of involvement, which are inherently associated with higher mortality rates (30). Although we noted a lower mortality rate from R. homothallicus infection on multivariate analysis, we cannot exclude residual confounding factors that could have resulted in improved survival with R. homothallicus infection. Also, we do not have a detailed evaluation of risk factors or genetic analysis to ascertain whether specific factors predispose persons to infection with R. homothallicus. Alhough the AFLP method is known for poor reproducibility, the results may be within an acceptable range when the test is repeated with the same batch of reagents, as we observed with our R. homothallicus isolates. Moreover, modification of the AFST protocol makes the results difficult to interpret and compare with published data.
In conclusion, our results show that R. homothallicus is an important agent of mucormycosis with epidemiologic and clinical significance. R. homothallicus may be less virulent or manifest earlier than R. arrhizus, thus resulting in better survival. Identifying R. homothallicus based on macroscopic and microscopic appearance is not difficult and should be emphasized. Most R. homothallicus isolates are uniformly susceptible to the commonly used antifungal agents to manage mucormycosis.
Additional information about clinical and mycologic characteristics of emerging mucormycosis agent Rhizopus homothallicus.
Acknowledgments
The Indian Council of Medical Research partially funded this work (grant no. ref no. AMR/149/2018-ECD-II).
Author contributions: conceptualization (S.M.R. and A.C.); methodology (S.S., R.K., N.P., H.C., A.P., and S.T.); formal analysis (S.S. and R.K.); investigations (H.C., A.P., R.K., and H.K.); resources (S.M.R. and A.C.); data curation (R.K., S.T., N.P., and V.M.); data interpretation (A.C., S.M.R., S.S., and V.M.); writing original draft (S.S.); writing review and editing (A.C., S.M.R., R.K., H.K., A.G., V.M., R.A., and R.A.); visualization (S.S.); and project administration (A.C.).
Biography
Dr. Rudramurthy is professor and heads the Mycology Division of the Medical Microbiology Department at the Postgraduate Institute of Medical Education and Research in Chandigarh, India. His primary research interests include epidemiology, antifungal resistance, and molecular diagnosis of fungal infections. Dr. Singh is an assistant professor at the Dr. B R Ambedkar State Institute of Medical Sciences in Mohali, India. Her primary research interests include medial mycology, fungal diagnostics, and invasive fungal diseases.
Footnotes
Suggested citation for this article: Rudramurthy SM, Singh S, Kanaujia R, Chaudhary H, Muthu V, Panda N, et al. Clinical and mycologic characteristics of emerging mucormycosis agent Rhizopus homothallicus. Emerg Infect Dis. 2023 Jul [date cited]. https://doi.org/10.3201/eid2907.221491
These first authors contributed equally to this article.
Current affiliation: Dr. B R Ambedkar State Institute of Medical Sciences, Mohali, India.
Current affiliation: Indian Council of Medical Research, New Delhi, India.
Current affiliation: Doodhadhari Burfani Hospital, Haridwar, India.
References
- 1.Prakash H, Chakrabarti A. Epidemiology of Mucormycosis in India. Microorganisms. 2021;9:523. 10.3390/microorganisms9030523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeong W, Keighley C, Wolfe R, Lee WL, Slavin MA, Kong DCM, et al. The epidemiology and clinical manifestations of mucormycosis: a systematic review and meta-analysis of case reports. Clin Microbiol Infect. 2019;25:26–34. 10.1016/j.cmi.2018.07.011 [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Pal N, Chander J, Sardana R, Mahajan B, Joseph N, et al. Mucormycosis caused by Syncephalastrum spp.: clinical profile, molecular characterization, antifungal susceptibility and review of literature. Clin Infect Pract. 2021;11:100074. 10.1016/j.clinpr.2021.100074 [DOI] [Google Scholar]
- 4.Chakrabarti A, Marak RS, Shivaprakash MR, Gupta S, Garg R, Sakhuja V, et al. Cavitary pulmonary zygomycosis caused by Rhizopus homothallicus. J Clin Microbiol. 2010;48:1965–9. 10.1128/JCM.01272-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokkayil P, Pandey M, Agarwal R, Kale P, Singh G, Xess I. Rhizopus homothallicus causing invasive infections: series of three cases from a single centre in North India. Mycopathologia. 2017;182:921–6. 10.1007/s11046-017-0153-5 [DOI] [PubMed] [Google Scholar]
- 6.Chakrabarti A, Ghosh A, Prasad GS, David JK, Gupta S, Das A, et al. Apophysomyces elegans: an emerging zygomycete in India. J Clin Microbiol. 2003;41:783–8. 10.1128/JCM.41.2.783-788.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chakrabarti A, Shivaprakash MR, Curfs-Breuker I, Baghela A, Klaassen CH, Meis JF. Apophysomyces elegans: epidemiology, amplified fragment length polymorphism typing, and in vitro antifungal susceptibility pattern. J Clin Microbiol. 2010;48:4580–5. 10.1128/JCM.01420-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel A, Agarwal R, Rudramurthy SM, Shevkani M, Xess I, Sharma R, et al. ; MucoCovi Network3. MucoCovi Network3. Multicenter epidemiologic study of coronavirus disease-associated mucormycosis, India. Emerg Infect Dis. 2021;27:2349–59. 10.3201/eid2709.210934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaur H, Kanaujia R, Rudramurthy SM. Rhizopus homothallicus: An emerging pathogen in era of COVID-19 associated mucormycosis. Indian J Med Microbiol. 2021;39:473–4. 10.1016/j.ijmmb.2021.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. ; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12:1500–24. 10.1016/j.ijsu.2014.07.014 [DOI] [PubMed] [Google Scholar]
- 11.Muraleedharan M, Panda NK, Angrish P, Arora K, Patro SK, Bansal S, et al. As the virus sowed, the fungus reaped! A comparative analysis of the clinico-epidemiological characteristics of rhino-orbital mucormycosis before and during COVID-19 pandemic. Mycoses. 2022;65:567–76. 10.1111/myc.13437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muthu V, Dhaliwal M, Sharma A, Nair D, Kumar HM, Rudramurthy SM, et al. Serum glucose-regulated protein 78 (GRP78) levels in COVID-19-associated mucormycosis: results of a case-control study. Mycopathologia. 2022;187:355–62. 10.1007/s11046-022-00645-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar H M, Sharma P, Rudramurthy SM, Sehgal IS, Prasad KT, Pannu AK, et al. Serum iron indices in COVID-19-associated mucormycosis: A case-control study. Mycoses. 2022;65:120–7. 10.1111/myc.13391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muthu V, Agarwal R, Patel A, Kathirvel S, Abraham OC, Aggarwal AN, et al. Definition, diagnosis, and management of COVID-19-associated pulmonary mucormycosis: Delphi consensus statement from the Fungal Infection Study Forum and Academy of Pulmonary Sciences, India. Lancet Infect Dis. 2022;22:e240–53. 10.1016/S1473-3099(22)00124-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Hoog GS, Guarro J, Gene J, Ahmed SA, Al-Hatmi AMS, Figueras MJ, et al. Atlas of clinical fungi. 4th edition. Hilversum: Foundation Atlas of Fungi; 2020. Washington: ASM Press; 2001. [Google Scholar]
- 16.Shivaprakash MR, Appannanavar SB, Dhaliwal M, Gupta A, Gupta S, Gupta A, et al. Colletotrichum truncatum: an unusual pathogen causing mycotic keratitis and endophthalmitis. J Clin Microbiol. 2011;49:2894–8. 10.1128/JCM.00151-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White TJ, Bruns S, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. London: Academic Press; 1990. p. 315–22. [Google Scholar]
- 18.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. 3rd edition. 2017. Nov 30 [cited 2018 Aug 2]. https://clsi.org/standards/products/microbiology/documents/m38
- 20.Prakash H, Ghosh AK, Rudramurthy SM, Paul RA, Gupta S, Negi V, et al. The environmental source of emerging Apophysomyces variabilis infection in India. Med Mycol. 2016;54:567–75. 10.1093/mmy/myw014 [DOI] [PubMed] [Google Scholar]
- 21.Compain F, Aït-Ammar N, Botterel F, Gibault L, Le Pimpec Barthes F, Dannaoui E. Fatal pulmonary mucormycosis due to Rhizopus homothallicus. Mycopathologia. 2017;182:907–13. 10.1007/s11046-017-0151-7 [DOI] [PubMed] [Google Scholar]
- 22.Prakash H, Ghosh AK, Rudramurthy SM, Singh P, Xess I, Savio J, et al. A prospective multicenter study on mucormycosis in India: Epidemiology, diagnosis, and treatment. Med Mycol. 2019;57:395–402. 10.1093/mmy/myy060 [DOI] [PubMed] [Google Scholar]
- 23.Patel A, Kaur H, Xess I, Michael JS, Savio J, Rudramurthy S, et al. A multicentre observational study on the epidemiology, risk factors, management and outcomes of mucormycosis in India. Clin Microbiol Infect. 2020;26:944.e9–15. 10.1016/j.cmi.2019.11.021 [DOI] [PubMed] [Google Scholar]
- 24.Kaerger K, Schwartze VU, Dolatabadi S, Nyilasi I, Kovács SA, Binder U, et al. Adaptation to thermotolerance in Rhizopus coincides with virulence as revealed by avian and invertebrate infection models, phylogeny, physiological and metabolic flexibility. Virulence. 2015;6:395–403. 10.1080/21505594.2015.1029219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biswal M, Gupta P, Kanaujia R, Kaur K, Kaur H, Vyas A, et al. Evaluation of hospital environment for presence of Mucorales during COVID-19-associated mucormycosis outbreak in India - a multi-centre study. J Hosp Infect. 2022;122:173–9. 10.1016/j.jhin.2022.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh AK, Singh R, Reddy S, Singh S, Rudramurthy SM, Kaur H, et al. Evaluation of environmental Mucorales contamination in and around the residence of COVID-19-associated mucormycosis patients. Front Cell Infect Microbiol. 2022;12:953750. 10.3389/fcimb.2022.953750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. 10.1086/432579 [DOI] [PubMed] [Google Scholar]
- 28.Cornely OA, Alastruey-Izquierdo A, Arenz D, Chen SCA, Dannaoui E, Hochhegger B, et al. ; Mucormycosis ECMM MSG Global Guideline Writing Group. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19:e405–21. 10.1016/S1473-3099(19)30312-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muthu V, Rudramurthy SM, Chakrabarti A, Agarwal R. Epidemiology and pathophysiology of COVID-19-associated mucormycosis: India versus the rest of the world. Mycopathologia. 2021;186:739–54. 10.1007/s11046-021-00584-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muthu V, Agarwal R, Dhooria S, Sehgal IS, Prasad KT, Aggarwal AN, et al. Has the mortality from pulmonary mucormycosis changed over time? A systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:538–49. 10.1016/j.cmi.2020.12.035 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional information about clinical and mycologic characteristics of emerging mucormycosis agent Rhizopus homothallicus.


