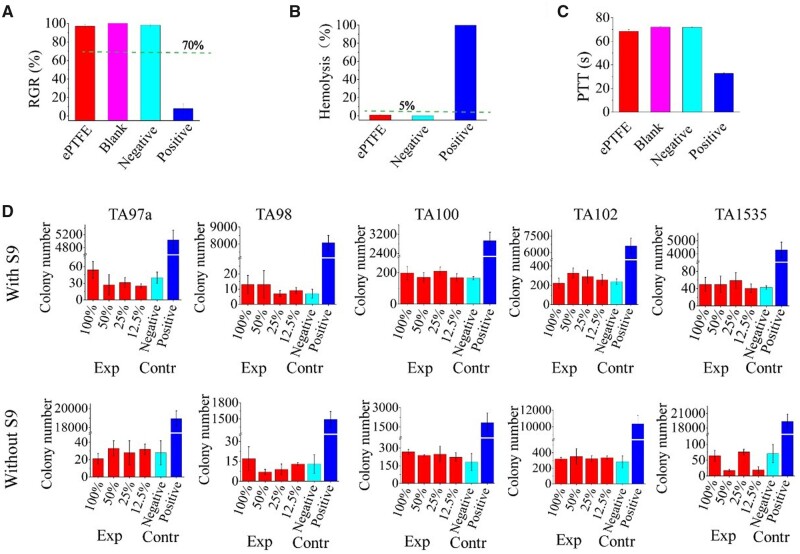
Figure 8.
In vitro biocompatibility test and bacterial reverse mutation tests of sintered ePTFE membranes with five test strains (Salmonella typhimurium TA97a, TA98, TA100, TA102 and TA1535). (A) Cytotoxicity test of the sintered ePTFE membrane. SDS, HDPE and culture media are set as the positive, negative and blank controls, respectively. The negative control sample, positive control sample, blank control and sintered ePTFE membrane were extracted under the same extraction conditions (37°C, 24 h). The dashed line indicates the criteria of the corresponding ISO 10993.5 of in vitro cell viability for a medical device. (B) Hemolysis tests of the sintered ePTFE membrane. Normal saline and distilled water were used as negative and positive controls, respectively. The dashed line indicates the criteria of acceptable hemolysis for a medical device. (C) Coagulation test of the sintered ePTFE membrane. The negative group was HDPE, which does not activate coagulation. Glass beads were used as positive material with a shortened PTT. The blank group was untreated platelet plasma that provides a normal background PTT in the coagulation study. (D) Bacterial reverse mutation tests on the sintered ePTFE membrane. The DMSO extract from sintered ePTFE membrane was used as the experimental group, DMSO as the negative control group and the mutagen as the positive control group. Among them, 100% refers to the extract stock solution, 50%, 25%, 12.5% refers to the gradient dilution of the extract stock solution.