Abstract
Diabetic patients frequently experience neuropathic pain, which currently lacks effective treatments. The mechanisms underlying diabetic neuropathic pain remain unclear. The anterior cingulate cortex (ACC) is well-known to participate in the processing and transformation of pain information derived from internal and external sensory stimulation. Accumulating evidence shows that dysfunction of microglia in the central nervous system contributes to many diseases, including chronic pain and neurodegenerative diseases. In this study, we investigated the role of microglial chemokine CXCL12 and its neuronal receptor CXCR4 in diabetic pain development in a mouse diabetic model established by injection of streptozotocin (STZ). Pain sensitization was assessed by the left hindpaw pain threshold in von Frey filament test. Iba1+ microglia in ACC was examined using combined immunohistochemistry and three-dimensional reconstruction. The activity of glutamatergic neurons in ACC (ACCGlu) was detected by whole-cell recording in ACC slices from STZ mice, in vivo multi-tetrode electrophysiological and fiber photometric recordings. We showed that microglia in ACC was significantly activated and microglial CXCL12 expression was up-regulated at the 7-th week post-injection, resulting in hyperactivity of ACCGlu and pain sensitization. Pharmacological inhibition of microglia or blockade of CXCR4 in ACC by infusing minocycline or AMD3100 significantly alleviated diabetic pain through preventing ACCGlu hyperactivity in STZ mice. In addition, inhibition of microglia by infusing minocycline markedly decreased STZ-induced upregulation of microglial CXCL12. Together, this study demonstrated that microglia-mediated ACCGlu hyperactivity drives the development of diabetic pain via the CXCL12/CXCR4 signaling, thus revealing viable therapeutic targets for the treatment of diabetic pain.
Keywords: diabetic neuropathic pain, anterior cingulate cortex, microglia, glutamatergic neurons, microglia-neuron communication, CXCL12/CXCR4 signaling, minocycline, AMD3100
Introduction
Diabetes is a metabolic disease characterized by chronic hyperglycemia, and neuropathic pain is one of the main symptoms of diabetic neuropathy, affecting 25%–50% of patients [1]. The typical features of diabetic pain are burning, tingling, and knife cut-like or electric shock-like pain, and severe cases require amputation [2]. The pathophysiological mechanisms underlying diabetic pain remain elusive, and development of an effective treatment for diabetic pain remains a major challenge in the field.
Microglial cells are widely considered to be the resident immune cells in the brain, functioning as active surveyors of the extracellular environment in both healthy and disordered brains [3, 4]. Accumulating evidence shows that dysfunction of microglia in the central nervous system contributes to many diseases, including chronic pain and neurodegenerative diseases [5, 6]. For example, microglial hyperactivation has been reported in both spinal cord and supraspinal levels in inflammatory and neural injury animal pain models in which the microglia inhibitor minocycline attenuated nociceptive behavior [7–10]. Given that neuropathic pain frequently accompanies diabetes, whether and how microglia contribute to the development of diabetic pain is a timely topic for research.
Communication between neurons and microglia is crucial for optimal regulation of behavior and physiology, and the dysfunction of this communication results in containment or aggravation of disease progression. Microglia are important regulators of neuronal connections, excitability, development, and plasticity [11–14]. Pathological neuronal plasticity in the nervous system contributes to the central sensitization underlying pain. Many brain regions, including the anterior cingulate cortex (ACC), thalamus, somatosensory cortex, amygdala, and hippocampus play functional roles in regulating pain-associated brain networks [15–17]. Specifically, functional magnetic resonance imaging (fMRI) found that gray matter density decreased in multiple brain regions of diabetic patients with neuropathic pain, including the superior temporal gyrus, left corner gyrus, left temporal gyrus, middle frontal gyrus, somatosensory cortex, ACC, and thalamus, compared with healthy volunteers [18–20]. Hyperactivity of thalamic ventral posterolateral neurons was previously shown using a rat model of STZ-induced diabetic pain [20]. Highly processed, polymodal information that reaches the thalamus can be projected to the cortex regions, such as the ACC, which is well-known to participate in internal and external sensory stimulation and in the processing and transformation of pain information [21, 22]. Both peripheral inflammation and nerve damage can activate ACC neurons [23, 24], e.g., inhibitory synapse loss and increased neuronal excitability of ACC pyramidal neurons, in animal models of chronic pain [25, 26]. Investigation by fMRI showed that the functional connection between the ventrolateral periaqueductal gray matter and the ACC is enhanced in patients with diabetes [27]. However, it remains unknown whether the dysfunction of microglia and their interactions with neurons in the ACC are involved in the pain resulting from diabetes.
In the present study, we combined three-dimensional reconstruction, in vivo multi-tetrode electrophysiological and fiber photometric recordings to demonstrate that upregulation of microglial CXCL12 provoked hyperactivity in glutamatergic neurons by acting on CXCR4 in the ACC, consequently leading to the occurrence of pain sensitization in diabetic mice. Chemical manipulation of CXCL12/CXCR4 signaling significantly affected the pain threshold in these mice. This study thus provides a plausible and experimentally tractable framework to understand the molecular, cellular basis of diabetic pain, and implicates new therapeutic targets for treatment of diabetic pain.
Materials and methods
Animals
All of the animal experiments were approved by the Animal Care and Use Committee of the University of Science and Technology of China. We used male mice aged 8–10 weeks for all experimental research, including C57BL/6 J (Beijing Vital River Laboratory Animal Technology Co., Ltd, China), CaMKII-Cre, and Ai14 (RCL-tdT) mice (Charles River or Jackson Laboratories, USA). Mice were group-housed five per cage, except for diabetic mice housed 2–3 per cage and the mice implanted with tetrodes housed one per cage; all mice had ad libitum access to food and water. They were housed at a stable temperature (23–25 °C) with a 12-h light/dark cycle (lights on from 7:00 a.m. to 7:00 p.m.).
Animal model of diabetes-associated pain
To induce diabetes, a single dose of 180 mg/kg STZ was intraperitoneally injected into mice that were fasted for at least four hours but were provided water. Before injection, the STZ was quickly dissolved in sodium citrate buffer (pH 4.5, 50 mM) to a final concentration of 20 mg/ml, and the injection was completed within five minutes. An equal volume of sodium citrate buffer (vehicle) was injected into the age-matched control mice along with diabetic animals. Mice were provided free food and 10% (w/v) sucrose water. The 10% sucrose water was replaced with regular water after 48 h. A glucometer (Roche, Switzerland) was used to test the blood glucose levels in the tail vein blood samples of mice fasted for 6 h to confirm that STZ treatment induced hyperglycemia. The mice with blood glucose levels more than 16.7 mmol/L were selected and used in this study. At the end of each experiment, blood glucose levels and body weight were measured.
von Frey filament test
Calibrated von Frey filaments were used for testing the mechanical withdrawal threshold of mice. To accustom them to the testing environment, mice were individually placed in a transparent plastic chamber on a wire mesh grid at least 30 min. Then we tested the withdrawal threshold of the planta using von Frey filaments on the middle of the plantar surface of the hindpaws or forepaws. The pressure of the von Frey filament was increased gradually. A positive response was considered when a mouse withdrew or licked its paw. The withdrawal threshold was tested every 10 min and the mean withdrawal threshold was calculated from three applications. The experimenters were blinded to group identity during the experiment and quantitative analyses. In this study, pain threshold is determined using the left hindpaw, as previously described [28–30].
Immunohistochemistry and imaging
Mice were deeply anesthetized by an intraperitoneal injection with pentobarbital sodium, and sequentially perfused with ice-cold saline followed by 4% (w/v) paraformaldehyde (PFA). The brains were post-fixed in 4% PFA at 4 °C overnight and then incubated in 30% (w/v) sucrose until they sank. For immunofluorescence, 40 µm coronal sections were cut using a cryostat (Leica CM1860). The sections were incubated in blocking buffer (PBS containing 0.3% (v/v) Triton X-100 and 10% donkey serum) for 1 h at room temperature, and incubated with primary antibodies, including anti-Iba1 (1:500, rabbit, Woka and goat, Abcam), anti-MHCII (1:500, mouse, Abcam), anti-CXCL12 (1:60, mouse, R&D Systems), anti-CXCR4 (1:50, rat, R&D Systems) and anti-Glutamate (1:500, rabbit, Sigma Aldrich) at 4 °C for 24 h. The sections were washed with PBS three times, and incubated with the corresponding fluorophore-conjugated Alexa-Fluor 488, Alexa-Fluor 594 and Alexa-Fluor 647 secondary antibodies (Thermo Fisher) for 2 h at room temperature. Site images expressing GCaMP6f, hM4D(Gi) and ChR2 were stained by DAPI. Fluorescence signals were visualized using Zeiss LSM710 and LSM880 microscopes. Imaris 9.2 (Bitplane, Zurich, Switzerland) was used for Iba1 three-dimensional rendering and analysis. ImageJ software was used for Iba1, MHCII, CXCL12 and CXCR4 expression analysis; the slices randomly picked from per mouse were imaged and quantified for three mice per group. The mice used for immunofluorescence were pseudo-randomly assigned to the experimental group and the control group. Further analyses such as analysis of cell counts and colocalization were conducted using ImageJ software by an observer blind to condition.
Gait imaging
Gait imaging acquisition and imaging analysis of mice were conducted using the DigiGaitTM Imaging System (Mouse Specifics, Inc., USA). In the training paradigm, mice were adapted in a transparent treadmill once every day a total of three times (5 min per session). On experiment day, the mouse was placed on the transparent treadmill and the imaging system was used to record the running state of the mouse. DigiGait Analyses software was used for the statistical analysis of mice in a uniform speed running state within 2 s.
Virus injection
Prior to surgery, a stereotactic frame (RWD, Shenzhen, China) was used to fix the mice under anesthesia by an intraperitoneal injection of pentobarbital (20 mg/kg). A heating pad was used to maintain the core body temperature of mice at 36 °C. Depending on the viral titer and expression strength, a volume of 100–200 nl virus was injected into the ACC at a rate of 30 nl/min through calibrated glass microelectrodes connected to an infusion pump (micro 4, WPI, USA). The pipette remained in the injection site for 5 min at the end of infusion to avoid virus overflow. The coordinates were defined as dorso-ventral (DV) from the brain surface, medio-lateral (ML) from the midline and anterior-posterior (AP) from bregma (in mm).
For fiber photometry, the rAAV-CaMKIIa-GCaMP6f-WPRE-pA (AAV-CaMKIIa-GCaMP6f, AAV2/9, 2.53 × 1012 vg/ml, 180 nl) virus was delivered into the ACC (AP, + 0.50 mm; ML, −0.25 mm; DV, −1.08 mm) of C57BL/6 J mice. For chemogenetic manipulation, Cre-dependent virus rAAV-Ef1α-DIO-hM4D(Gi)-mCherry-WPRE-pA (AAV-DIO-hM4Di-mCherry, AAV2/9, 3.69 × 1013 vg/ml, 150 nl) was delivered into the ACC of CaMKII-Cre mice, three weeks after viral injection, with an intraperitoneal injection of CNO (5 mg/kg, Sigma-Aldrich, USA) 30 min before the behavioral tests [31]. The rAAV-Ef1α-DIO-mCherry-WPRE-pA (AAV-DIO-mCherry, AAV2/8, 8.93 × 1012 vg/ml) virus was used as the control. For optogenetic manipulation, the rAAV-Ef1α-DIO-hChR2 (H134R)-mCherry-WPRE-pA (AAV-DIO-ChR2-mCherry, AAV2/9, 1.63 × 1013 vg/ml, 200 nl) virus was used. All viruses were packaged by BrainVTA (Wuhan, China). All mice were transcardially perfused with ice-cold 0.9% saline followed by 4% PFA. Images of the signal expression were acquired with a confocal microscope Zeiss LSM710 or LSM880 microscope. Animals with missed injections were excluded.
Fiber photometry
Following AAV-CaMKIIa-GCaMP6f virus injection, an optical fiber (200 mm O.D., 0.37 numerical aperture (NA); Inper, Hangzhou) was placed in a ceramic ferrule and inserted towards the ACC through the craniotomy. The ceramic ferrule was supported with three skull-penetrating M1 screws and dental acrylic. After the virus was expressed for three weeks, the optical-fiber-based Ca2+ signals of the ACCGlu neuron population were detected by a custom-built setup (Thinkertech, Nanjing, China) during a pain threshold test. To excite GCaMP6f fluorescence, a 488-nm LED light beam (30 μW, Cree XPE LED, Coherent as a driver) was reflected by a dichroic mirror (MD498, Thorlabs) and coupled to an optical commutator (Doris Lenses) after focusing through a 20× objective lens (0.4 NA, Olympus). The light intensity at the tip of the fiber was 0.03 mW. Bandpass filtered (MF525–39, Thorlabs) light was collected by a photomultiplier tube (H10721–210, Hamamatsu) and then converted from the photomultiplier tube current output to voltage signals by an amplifier (C7319, Hamamatsu). A real-time processor including a Power 1401 digitizer and Spike2 software (CED, Cambridge, UK) was used to record the converted signal as a digitized signal. Ca2+ signals were sampled at 100 Hz through customized acquisition software written in LabView (National Instrument, USA). Behavioral videos were recorded with a video camera. Behavioral videos and neuronal Ca2+ signals were recorded simultaneously. Calcium signal analysis was conducted using Matlab toolkit OpSignal. For the chart or heatmaps of changes in Ca2+ signals, the ΔF/F (%) values were calculated as (Fsignal-Fbaseline)/Fbaseline × 100, where Fbaseline is the mean of GCaMP6f signal for 2 s before time zero (von Frey stimulus initiation) and Fsignal is the GCaMP6f signal for the entire session. A custom MATLAB script developed by ThinkerTech was used to form typical traces.
Brain slice preparation
Acute brain slices were prepared as previously described [32]. Mice were deeply anesthetized by an intraperitoneal injection of pentobarbital sodium (2% w/v) and intracardially perfused with ice-cold oxygenated modified N-methyl-D-glucamine artificial cerebrospinal fluid (NMDG ACSF) that contained (in mM) 30 NaHCO3, 2.5 KCl, 93 NMDG, 1.2 NaH2PO4, 25 glucose, 2 thiourea, 20 HEPES, 3 Na-pyruvate, 10 MgSO4, 5 Na-ascorbate and 0.5 CaCl2, 3 glutathione [33] (pH: 7.3–7.4, osmolarity: 300–310 mOsm/kg). Coronal slices (300 µm) that contained the ACC were sectioned on a vibrating microtome (VT1200s, Leica, Germany) at a rate of 0.18 mm/s. The sectioned brain slices were initially incubated in NMDG ACSF for 12–15 min at 33 °C, followed by N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES) ACSF that contained (in mM) 2.4 CaCl2, 3 KCl, 3 HEPES, 129 NaCl, 1.2 KH2PO4, 1.3 MgSO4, 20 NaHCO3 and 10 glucose (pH: 7.3–7.4, osmolarity: 300–310 mOsm/kg) for at least 1 h at 28 °C. The brain slices were recorded after 2 h of incubation in HEPES containing AMD3100 (10 µM), and recorded after 0.5 h of incubation in HEPES containing CXCL12 (0.5 ng/ml). For whole cell recording, we transferred the brain slices to a slice chamber (Warner Instruments, USA) continuously perfused with standard ACSF solution (28 °C) that contained (in mM) 3 KCl, 10 glucose, 3 HEPES, 129 NaCl, 1.3 MgSO4, 2.4 CaCl2, 20 NaHCO3 and 1.2 KH2PO4 (pH: 7.3–7.4, osmolarity: 300–310 mOsm/kg) at a rate of 2.5–3 ml/min. An in-line solution heater (TC-344B, Warner Instruments, USA) was used to maintain the temperature of the standard ACSF.
Whole-cell patch-clamp recordings
To visualize neurons in the ACC region, we used a water-immersion objective (×40) on an upright microscope (BX51WI, Olympus, Japan), which was equipped with interference contrast (IR/DIC) and an infrared camera connected to the video monitor. Whole-cell patch-clamp recordings were obtained from visually identified ACC neurons. A four-stage horizontal puller (P1000, Sutter Instruments, USA) was used to obtain patch pipettes that were pulled from borosilicate glass capillaries (outer diameter: 1.5 mm, VitalSense Scientific Instruments Co., Ltd., Wuhan, China). The signals were acquired after being digitized at 10 kHz and low-pass filtered at 2.8 kHz via a Multiclamp 700B amplifier. The data were collected from the neurons with the appropriate input resistance (more than 100 MΩ) and series resistance (less than 30 MΩ). Experimental recording was immediately terminated when the series resistance changed by more than 20% during recording. The current-evoked firing was recorded in current-clamp mode (I = 0 pA) using pipettes filled with potassium gluconate-based internal solution resistance containing (in mM): 130 K-gluconate, 10 HEPES, 2 MgCl2, 5 KCl, 0.6 EGTA, 2 Mg-ATP and 0.3 Na-GTP (pH: 7.2, osmolality: 285–290 mOsm/kg). The threshold current of the action potential was defined as the minimum current to elicit an action potential.
Surgical implantation of tetrode/optrode
Prior to surgery, a stereotactic frame (RWD, Shenzhen, China) was used to fix the mice under anesthesia by an intraperitoneal injection of pentobarbital (20 mg/kg). A heating pad was used to maintain the core body temperature of mice at 36 °C. A homemade screw-driven microdrive was implanted on the right side of the ACC. The microdrive carried 4–8 adjustable tetrode arrays that can record simultaneously from multiple neurons at the same time. The tetrode was made of four twisted fine platinum/iridium wires (12.5 μm diameter, California Fine Wire). Signals were recorded after at least 3 days of recovery from the surgery, and mice were habituated to the cables connected to the electrode on their heads before recording. For the purpose of optogenetic tagging of glutamate neurons, tetrodes were replaced with optrodes consisting of one optic fiber (200 µm, Inper) surrounded by multiple tetrodes, with the tip protruding 200 μm beyond the fiber (Fig. S5b). Wires were soldered to a 16-channel or 32-channel connector (Senon, Taiwan, China). The whole implant was fixed to the skull with four skull-penetrating M1 screws and dental acrylic. Mice were then singly-housed.
Optogenetic identification of glutamate neurons
For in vivo optogenetic tagging of ACC glutamatergic neurons, the AAV-DIO-ChR2-mCherry virus was unilaterally delivered into the right ACC of CaMKII-Cre mice [34]. Three weeks later, optrodes were implanted into the ACC with identical coordinates. For optical identification of ACCGlu neurons, blue light pulses (473 nm, 20 Hz, 2 ms duration, 0.08–1.35 mW at fiber tip) were delivered at the end of each recording session. Units that exhibited time-locked spiking with high reliability (>90%), low jitter (<2 ms) and short first-spike latency (<3 ms) upon light pulse illumination were considered as light responsive. Only when the waveforms of spontaneous and laser-evoked spikes were highly similar (correlation coefficient >0.9), were they considered to originate from the same neuron.
In vivo electrophysiology recording
For chronic extracellular recording, the subject mouse was placed in a cylindrical box wrapped with copper mesh to allow it to move freely without any interference, and multichannel electrical signals were recorded during this period. Recording electrodes were attached to a 16-channel or a 32-channel headstage, and neuronal signals were filtered at a bandwidth of 300–5000 Hz and amplified. Neurostudio software (Jiangsu Brain Medical Technology, China) was used to store data of neuronal signals. Offline Sorter 4 (Plexon, USA) containing a sorting method of a T-Dis E-M algorithm was used for spike sorting. Neuroexplorer 4 (Nex Technologies, USA) was used to calculate the firing rates of sorted units. An unsupervised clustering algorithm based on a κ-means method was used to classify well-isolated units into wide-spiking putative pyramidal neurons or narrow-spiking putative interneurons. The analysis was based on the three-dimensional space defined by each neuron’s half valley width, half-spike width (trough to peak duration) and the mean firing rate at baseline [35]. Spikes with longer half valley width, half-spike width, and lower firing rate were classified as pyramidal neurons; almost all of these spikes in the ACC are considered to be glutamatergic neurons [36]. Animals outside of the desired location of the tetrode were excluded.
In vivo pharmacological approach
A cannula (internal diameter 0.25 mm, RWD) was initially implanted into the ACC of an anesthetized mouse that had been immobilized in a stereotactic frame. The cannula was supported with three skull-penetrating M1 screws and dental acrylic. An internal stainless-steel injector attached to a 10 μl syringe (Hamilton) and an infusion pump (micro 4, WPI, USA) was inserted into the guide cannula and used to infuse minocycline (5 μg/200 nl/side/day) [37] or AMD3100 (10 μM/500 nl/side) into the bilateral ACC at a flow rate of 200 nl/min. Mice were administered with minocycline or ACSF starting at the fifth week after STZ treatment until the seventh week. Similarly, CXCL12 (0.2 ng /400 nl/side) or vehicle solution (ACSF, 400 nl) was injected into the right ACC. The injector was slowly withdrawn 2 min after the infusion and the pain testing was performed roughly 0.5 h after the infusion. The mice were allowed at least 10 d for recovery before injections to minimize stress during the pain testing, and the mice with missed injections were excluded from the study.
Label-free protein mass spectrometry
Proteins were extracted using RIPA lysis buffer (150 mM NaCl, 50 mM Tris-HCl (pH 7.5), 1% NP40, 0.5% deoxycholate, 0.1% SDS with protease inhibitor). A BCA Protein Assay Kit (Thermo Scientific) was used for protein quantification. Proteins were resolved by SDS-PAGE under reducing conditions and digested by trypsin. The digested samples were analyzed by LC-MS/MS, followed by database query and quantitative protein significant difference analysis. STRING database was used for protein network interaction analysis.
Statistical analysis and drugs
GraphPad Prism 9 (GraphPad Software, Inc., USA) and OriginPro 2018 software (OriginLab Corporation, USA) were used for the statistical analyses and graphing. Offline analysis of the data obtained from electrophysiological recordings was conducted using Clampfit software version 10.6 (Axon Instruments, Inc., USA). Animals were randomly or pseudo-randomly assigned to experimental groups, which minimized the influence of other variables on the experimental outcome. We conducted statistical comparisons between two groups using paired or unpaired Student’s t-tests. One-way and two-way analysis of variance (ANOVA) and Bonferroni post hoc analyses were used in analyses with multiple experimental groups. Data are shown as individual values or expressed as the mean ± SEM, and significance levels are indicated as *P < 0.05, **P < 0.01, and ***P < 0.001, and not significant (n.s.). P values are not provided as exact values when they less than 0.0001. All statistical tests, significance analyses, number of individual experiments (n) and other relevant information for data comparison are specified in Supplementary Table 1. Unless otherwise stated, all drugs were purchased from Sigma-Aldrich (USA). CNO was obtained from MedChemExpress (China).
Results
Microglia are activated in the ACC of diabetic pain mice
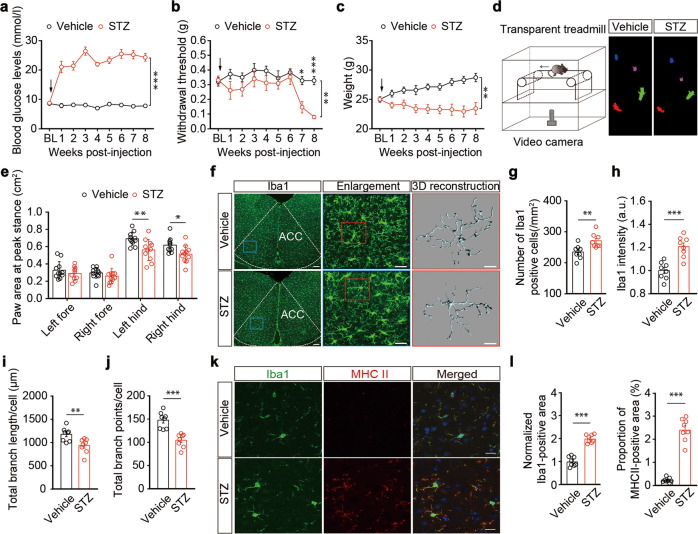
A mouse model of diabetes was established through intraperitoneal injection of streptozotocin (STZ) [38, 39]. Mice given a single injection of STZ showed significantly increased blood glucose concentrations at one week, and remained hyperglycemic thereafter (Fig. 1a). Additionally, STZ mice had higher water intake, food intake, and urine output than control vehicle (sodium citrate buffer)-treated mice (Fig. S1a). These mice exhibited a progressive hypersensitivity in response to von Frey tests; the pain threshold in both hindpaws of STZ mice was significantly lower than that in vehicle mice starting from the seventh week after STZ injection (Figs. 1b and S1b, c), which lasted at least until the 19th week (Fig. S1d), accompanied by decreased body weight (Figs. 1c and S1d). Furthermore, at the seventh week, the areas of the hindpaws of the STZ mice were significantly lower than those of the vehicle mice during the peak period, and showed a painful gait indicated by a DigiGaitTM Imaging System to examine the gait dynamics and posture (Figs. 1d, e and S1e). The diabetic pain mice were thus defined at seven weeks after STZ injection (STZ 7 W) in this study.
Fig. 1. Microglia are activated in STZ 7W mice.
Time course for STZ-induced changes in blood glucose (a), the left hindpaw pain threshold (b), and body weight (c) (n = 10 mice per group). Arrows indicate STZ or vehicle (sodium citrate buffer) injection on day 0. BL, baseline. d Experimental paradigms for mouse gait behavior tests (left). Physiological gait signals of STZ 7 W and vehicle mice (right). The arrow indicates the direction of conveyor belt movement. e Summarized data showing paw areas at peak stance for vehicle and STZ 7 W mice (n = 11 mice per group). f Representative images of Iba1 immunostaining and three-dimensional (3D) reconstruction of Iba1+microglia (gray) in the ACC (n = 8 slices from three mice per group). Scale bars, 100 μm (left), 30 μm (middle), 10 μm (right). Quantification of cell morphometry of Iba1+ microglia in the ACC. The STZ 7 W mice showed increased Iba1+ microglia cell numbers (g), higher overall Iba1+ intensity (h), decreased total process length (i), and fewer branch points of microglia (j) compared with vehicle mice (n = 8 slices from three mice per group). Representative images (k) and quantitative analyses (l) of immunostaining for Iba1 (left), MHC II (middle), and merged channels (right) in the ACC (n = 8 slices from three mice per group). Data are shown as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Two-way repeated-measures ANOVA with Bonferroni post hoc analysis for (a–c, e). A two-tailed unpaired Student’s t test was used for (g, h, i, j, and l)
Guided by previous reports that described disrupted microglial function in inflammatory and neural injury animal models [7, 8], we investigated whether microglia are also involved in diabetic pain. We stained brain slices with Iba1, a recognized marker for microglia (Fig. 1f), and found increased microglial numbers and Iba1 signals in the ACC of STZ 7 W mice relative to control mice (Fig. 1g, h). We subsequently analyzed the morphology of microglial cells, as this is known to correlate well with their activation status [40]. Semi-automatic quantitative morphometric three-dimensional measurements of microglia revealed significantly shorter processes and decreased branch points in STZ 7 W mice compared with control mice (Fig. 1i, j). In addition, immunostaining showed increased levels of the inflammatory marker MHCII in microglia of these mice; the reactivity of Iba1 was significantly correlated with the levels of MHCII (Fig. 1k, l). Notably, these differences were not observed in STZ 5 W mice, which displayed no pain sensitization (Fig. S2).
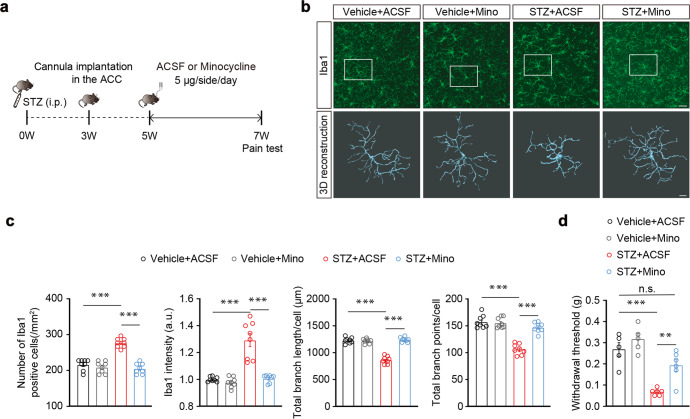
To examine the functional role of ACC microglia in the regulation of diabetes-associated pain, we inhibited ACC microglial activity based on injection minocycline (Mino) directly into the ACC. Minocycline is a selective inhibitor of microglia; specifically, mice were administered with minocycline or vehicle (saline) starting at the fifth week after STZ treatment (Fig. 2a). We found that minocycline administration inhibited STZ-induced activation of microglia (Fig. 2b, c). In addition, infusion of the ACC with minocycline significantly alleviated pain sensitization, and slightly decreased blood glucose levels in STZ 7 W mice compared with ACSF-treated mice, although it should be noted that minocycline-treated STZ 7 W mice remained in a diabetic state, and showed no effects on body weight (Fig. 2d and S3). These results suggest that pain sensitization is accompanied by ACC microglial activation in diabetic states.
Fig. 2. Effects of ACC infusion of minocycline on pain behaviors.
a Outline of the experimental procedure for minocycline treatment and behavioral tests. b Representative images of Iba1 immunostaining and three-dimensional (3D) reconstruction of Iba1+ microglia (gray) in the ACC (n = 8 slices from three mice per group). Scale bars, 30 μm (up), 8 μm (down). c Quantification of cell morphometry of Iba1+ microglia in the ACC. The STZ + Mino mice showed decreased Iba1+ cell numbers, lower overall Iba1+ intensity, increased total process length, and more branch points of microglia compared with STZ + ACSF-treated mice (n = 8 slices from three mice per group). d Pain threshold in STZ-treated mice with ACC infusion of minocycline (Mino) or ACSF for two weeks (n = 5–6 mice per group). Mino, minocycline. Data are shown as means ± SEM. ***P < 0.001; n.s., (not significant). A two-tailed unpaired Student’s t test was used for (c, d)
Microglia-mediated ACCGlu neuronal hyperactivity contributes to diabetic pain
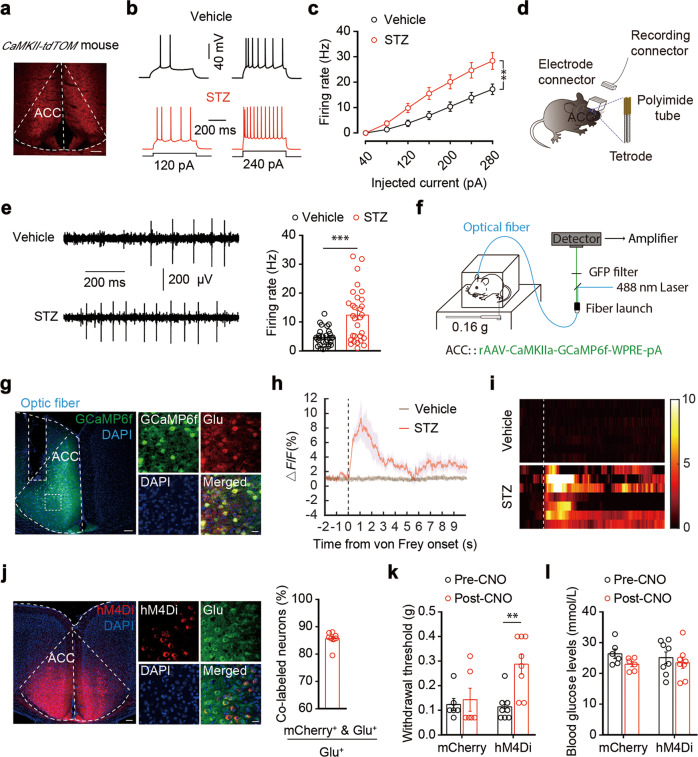
Microglia are known to participate in the regulation of neuronal activity for concomitant behavioral changes [41–43]. In light of this, we performed whole-cell recordings from visualized glutamate neurons in brain slices. To visualize glutamate neurons, Ca2+/calmodulin-dependent protein kinase II (CaMKII, an enzyme in glutamatergic neurons)-Cre mice were crossed with Ai14 (RCL-tdT) mice to produce transgenic mice with tdTomato-expressing glutamatergic neurons (CaMKII-tdTOM) (Fig. 3a). In response to a series of current injections, we found an increase in the spike number (Fig. 3b, c) and a decrease in rheobase (Fig. S4a), accompanied by increased membrane input resistance (Fig. S4b, c), in glutamatergic neurons of the ACC (ACCGlu) of STZ 7 W mice relative to control mice. However, no significant changes were detected in the resting membrane potentials or voltage threshold of STZ mice compared to vehicle control mice (Fig. S4d, e). In contrast, no difference was detectable in STZ 5 W mice (Fig. S4f–i).
Fig. 3. ACCGlu neuronal activity is enhanced in STZ 7W mice.
a A sample image of ACC neurons in a CaMKII-tdTOM mouse. Scale bar, 100 μm. Representative traces (b) and summarized data (c) of action potentials recorded from tdTOM+ neurons in the ACC slices from STZ 7 W and vehicle mice (n = 23 neurons from three mice per group). d Schematic illustration of an electrophysiological recording in the ACC of freely moving mice. Enlargement shows the multichannel tetrode. e Example recording of spontaneous spikes (left) and data (right) showing the ACCGlu neuronal firing rates in STZ 7 W and vehicle mice (n = 30 neurons from three mice per group). f Schematic diagram of the fiber photometric recording. Ca2+ transients were recorded from GCaMP6f-expressing ACC neurons of STZ 7 W and vehicle mice. g Representative images showing the AAV-CaMKIIa-GCaMP6f (GCaMP6f) injection site within the ACC (left). Scale bar, 100 µm. GCaMP6f-labeled neurons co-localized with glutamate immunofluorescence signals within the ACC (right). Scale bar, 20 µm. The average ΔF/F of GCaMP6f signal (h) and heatmap illustration (i) showed that Ca2+ signals in ACCGlu neurons were rapidly increased following 0.16-g von Frey stimuli in STZ 7 W mice compared to vehicle mice (n = 7 mice per group). The color scale at the right indicates ΔF/F (%). j Representative images showing the AAV-DIO-hM4Di (hM4Di) injection site within the ACC (left). Scale bar, 100 µm. Images (middle) and summarized data (right) show that hM4Di-labeled neurons within the ACC were mainly co-localized with glutamate immunofluorescence signals. Scale bar, 20 µm. Effects of chemogenetic inhibition of ACCGlu neurons on the pain threshold (k) and blood glucose levels (l) of STZ 7 W mice after ACC infusion of AAV-DIO-mCherry or AAV-DIO-hM4Di-mCherry (n = 8 mice per group). Data are shown as means ± SEM. **P < 0.01; ***P < 0.001. Two-way repeated-measures ANOVA with Bonferroni post hoc analysis was used for (c, k and l). A two-tailed unpaired Student’s t test was used for (e)
To confirm that ACCGlu neuronal activity was correlated with diabetic pain, we next employed in vivo multi-tetrode electrophysiological recordings. Specifically, mice were implanted with microdrives containing four to eight adjustable tetrodes aimed at the ACC, and spiking activities were recorded in freely moving mice (Fig. 3d). The well-isolated neurons were categorized into wide-spiking putative pyramidal neurons and narrow-spiking putative inhibitory interneurons according to spike features [35] (Fig. S5a). In addition, optogenetics allows precise in vivo identification of a genetically defined population of neurons [44]. We measured the spiking activity of glutamate neurons with optrodes consisting of one optic fiber surrounded by multiple tetrodes (Fig. S5b). For optical tagging of glutamate neurons, we delivered a Cre-dependent channelrhodopsin-2 (AAV-DIO-ChR2-mCherry) virus vector construct into the ACC of CaMKII-Cre mice, which revealed through immunofluorescence microscopy that the ChR2 signal was co-localized with the glutamate antibody (Fig. S5c). Blue light stimuli were applied at the end of each recording session; single units exhibited reliable light-evoked spikes (Fig. S5d), and glutamate neurons identified by the optrodes had distinguishable spike waveforms (Fig. S5e). In vivo multi-tetrode electrophysiological recordings showed that the firing rate of ACCGlu neurons increased in the freely moving STZ 7 W mice compared with controls (Figs. 3e, S5f).
To investigate whether ACCGlu neurons were sensitized to sub-threshold pain stimuli, fiber photometry recordings were performed in mice with ACC infusion of virally expressed fluorescent Ca2+ indicator GCaMP6f (rAAV-CaMKIIa-GCaMP6f-WPRE-pA); the GCaMP6f signal was co-localized with the glutamate antibody (Figs. 3f, g, and S6a). We found that the intensity of the calcium signal was rapidly increased by 0.16-g von Frey filament stimuli in STZ 7 W, but not in control mice (Fig. 3h, i and Supplementary Video 1).
Given these observations of a specific impact for increased ACCGlu neuronal activity on diabetic pain, we next investigated whether inhibition of ACCGlu neurons could alleviate the pain sensitization in STZ mice. Indeed, Cre-dependent expression of the chemogenetic inhibitory hM4Di in the ACC of CaMKIIa-Cre mice (Figs. 3j and S6b–d) followed by intraperitoneal injection of hM4Di ligand clozapine-N-oxide (CNO) significantly reversed the STZ-induced pain sensitization behavior, but had no effect on blood glucose or body weight (Figs. 3k, l, and S6e).
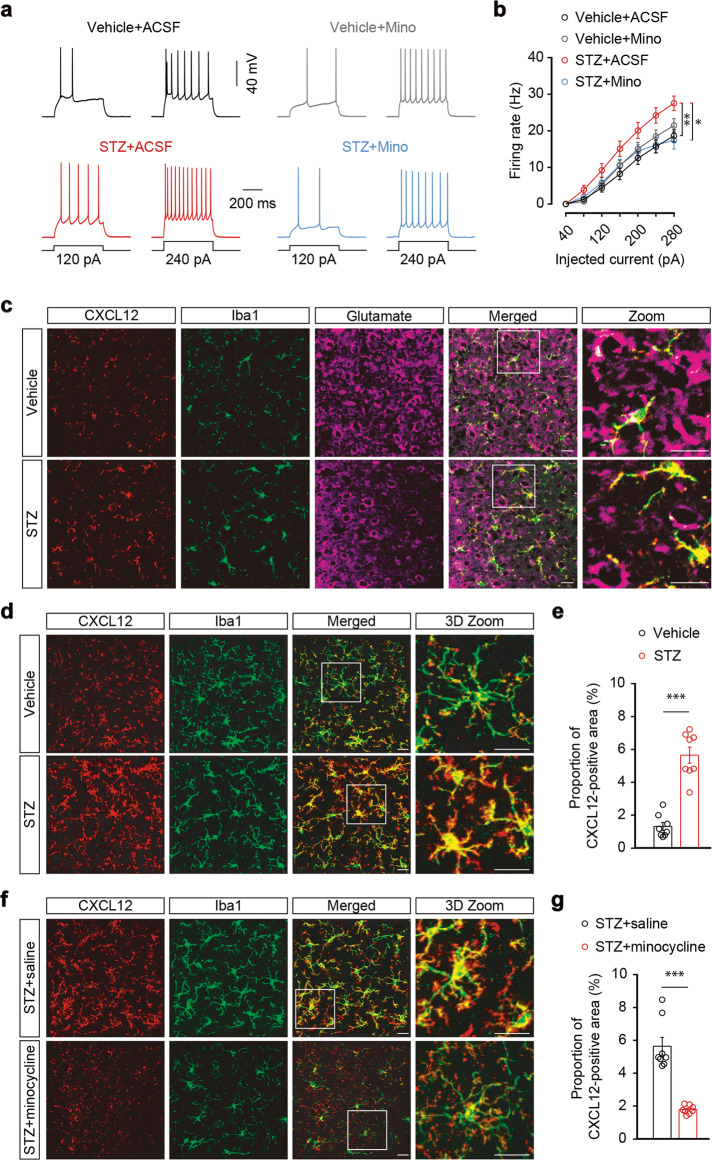
To investigate whether microglia contribute to the activation of ACCGlu neurons, we performed whole-cell recordings in brain slices from STZ 7 W mice treated with minocycline or saline starting from the fifth week. We found that minocycline administration inhibited the STZ-induced increase in evoked action potential firing of ACCGlu neurons (Fig. 4a, b). Together, these results suggest that enhanced activity of ACCGlu neurons, mediated by microglial activation, primes the development of diabetic pain.
Fig. 4. The expression of microglial CXCL12 is increased in STZ 7W mice.
Representative traces (a) and summarized data (b) of action potentials recorded from glutamate neurons in the ACC slices from STZ- and vehicle-administered mice treated with ACSF or Mino (n = 22 neurons from three mice per group). Mino, minocycline. c Representative two-dimensional immunostaining images of CXCL12 (red), Iba1 (green), and glutamate (purple) in the ACC of STZ 7 W and vehicle mice. Scale bars, 20 μm. d Representative three-dimensional (3D) immunostaining images of CXCL12 (red) and Iba1 (green) in the ACC of STZ 7 W and vehicle mice. Three-dimensional reconstruction with ~30 two-dimensional immunostaining images. Scale bars, 20 μm. e Summarized data obtained by 3D image reconstruction showing the proportions of the CXCL12-positive area in the ACC of mice, as indicated in (d) (n = 8 slices from three mice per group). f Representative 3D immunostaining images of CXCL12 (red) and Iba1 (green) in the ACC of STZ 7 W mice treated for 2 weeks with minocycline or saline starting from the fifth week after STZ injection. Scale bars, 20 μm. g Summarized data showing the proportions of CXCL12-positive area in the ACC of mice, as indicated in (f) (n = 8 slices from three mice per group). Data are shown as means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001. Two-way repeated-measures ANOVA with Bonferroni post hoc analysis was used for (b). A two-tailed unpaired Student’s t test was used for (e, g)
Microglial CXCL12-CXCR4 signaling governs ACCGlu neuronal hyperactivity
To examine the molecular basis of interactions between microglia and neurons, we performed label-free proteomics using mass spectrometry for quantitative protein profiling of ACC tissue from STZ and control mice. Analysis of the protein expression profiles revealed significant changes in STZ 7 W mice (Fig. S7a). Notably, we found that ubiquitin carboxyl-terminal hydrolase 14 (USP14) protein accumulation was lower in STZ 7 W mice than in control mice (Fig. S7a, b). USP14 is a proteasome-associated deubiquitinating enzyme relevant to hyperglycemia and insulin resistance [45] that participates in the degradation of CXC chemokine receptor 4 (CXCR4). Based on the close links that have been established between CXCR4 and inflammation, pain, and neuronal excitability [46, 47], we therefore focused our attention on USP14. De-ubiquitination of CXCR4 by USP14 is critical for both CXCR4 ligand C-X-C motif chemokine 12 (CXCL12)-induced CXCR4 degradation and chemotaxis [48]. Given the role of CXCL12 in neuropathic pain [49], we hypothesized that CXCL12-CXCR4 signaling may function in the development of diabetic pain.
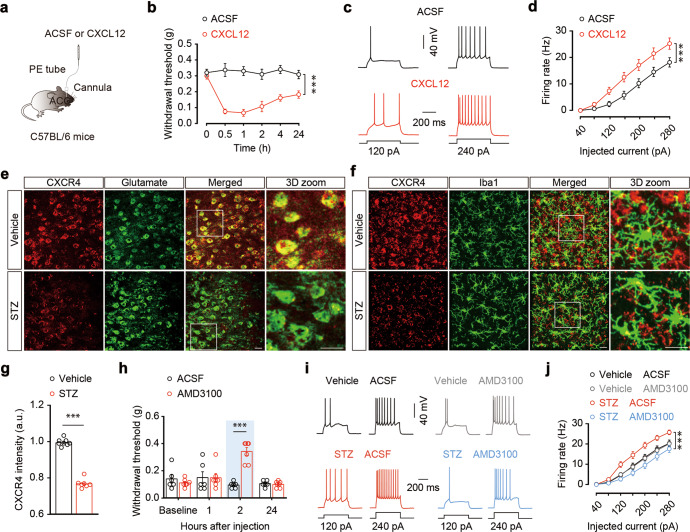
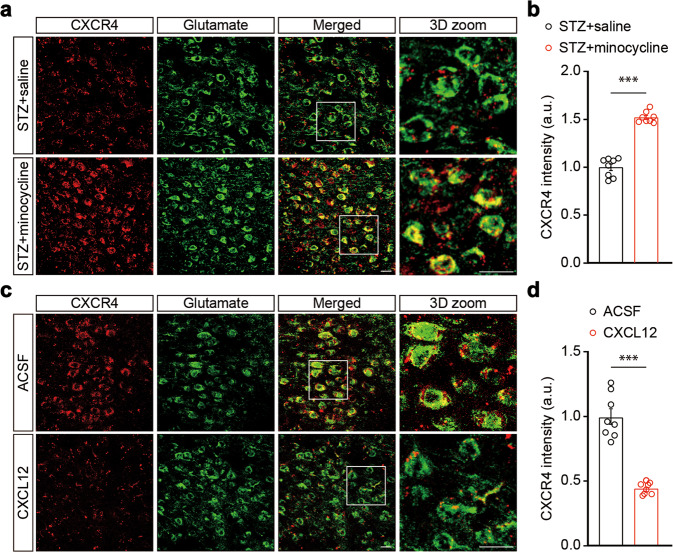
Immunostaining showed that CXCL12 was invariably co-labeled with Iba1, but not with the glutamate antibody in the ACC (Fig. 4c), suggesting microglia-specific expression of CXCL12. We then examined changes in the expression of CXCL12 in the current animal model and found a significant increase in the proportion of CXCL12-positive area in the ACC of STZ 7 W mice, compared with control mice (Fig. 4d, e). This change was reversed following minocycline administration starting from the fifth week after STZ treatment (Fig. 4f, g). In addition, a single ACC infusion of recombinant CXCL12 protein induced significant pain sensitization that lasted for at least 24 h (Fig. 5a, b), and an increase in the spike number of ACCGlu neurons in ACC slices from the naïve mice incubated with CXCL12 compared to those treated with ACSF (Fig. 5c, d). These results suggest that upregulation of CXCL12 in the ACC may be involved in diabetic pain.
Fig. 5. Inhibition of CXCR4 alleviates STZ 7W-induced pain sensitization.
a Schematic of right ACC injection with ACSF or CXCL12 in normal mice. b Measurement of mechanical pain hypersensitivity in normal mice with ACC infusion of CXCL12 or ASCF (n = 8 mice per group). Representative traces (c) and summarized data (d) of action potentials recorded from ACCGlu neurons in brain slices incubated with CXCL12 or ACSF for 0.5 h (n = 27 neurons from three mice per group). e Representative immunostaining images of CXCR4 (red) and glutamate (green) in the ACC of STZ 7 W and vehicle mice. Scale bars, 20 μm. f Representative immunostaining images of CXCR4 (red) and Iba1 (green) in the ACC of STZ 7 W and vehicle mice. Scale bars, 20 μm. g Summarized data showing the intensity of CXCR4 signals in mice, as indicated in (e) (n = 7–8 slices from three mice per group). h Effects of ACC infusion of AMD3100 or ACSF on pain threshold in STZ 7 W mice (n = 6–7 mice per group). Representative traces (i) and summarized data (j) of action potentials recorded from ACCGlu neurons in brain slices incubated with AMD3100 or ACSF for 2 h from STZ 7 W or vehicle mice (n = 21–26 neurons from three mice per group). Data are shown as means ± SEM. ***P < 0.001. Two-way repeated-measures ANOVA with Bonferroni post hoc analysis was used for (b, d, h, j). A two-tailed unpaired Student’s t test was used for (g)
CXCL12 can affect the expression of CXCR4 in response to different pathological conditions [50, 51], and was found to be involved in cancer, as well as in inflammatory and neurodegenerative disorders [52–54]. We then investigated the expression and distribution of CXCR4 and found that it was co-labeled with the glutamate antibody in the ACC, while in contrast, we rarely observed CXCR4 co-labeling with Iba1; the CXCR4 intensity in the ACC was significantly decreased in STZ 7 W mice compared with control mice (Fig. 5e–g). In addition, we found that ACC infusion with a selective antagonist of CXCR4, plerixafor (AMD3100), significantly alleviated pain sensitization in STZ 7 W mice compared with ACSF-treated mice (Fig. 5h), but had no significant effects on blood glucose levels or body weight (Fig. S8a). Furthermore, AMD3100 administration prevented the STZ-induced increase in evoked action potential firing of ACCGlu neurons (Fig. 5i, j), whereas it had no effects on microglial activation status (Fig. S8b, c).
Notably, the expression of CXCR4 in ACCGlu neurons increased in STZ 7 W mice treated with minocycline compared with saline (Fig. 6a, b). In contrast, the expression of CXCR4 decreased in mice treated with CXCL12 compared with ACSF (Fig. 6c, d). These results suggest that microglial activation may upregulate CXCL12-CXCR4 signaling, thereby leading to hyperactivity of ACCGlu neurons and ultimately resulting in the development of diabetic pain (Fig. S9).
Fig. 6. CXCL12 reduced neuronal CXCR4 in the ACC.
a Representative 3D immunostaining images of CXCR4 (red) and glutamate (green) in the ACC of STZ 7 W mice treated with saline or minocycline starting from the fifth week after STZ injection. Scale bars, 20 μm. b Summarized data showing the signal intensity of CXCR4 in mice, as indicated in (a) (n = 8 slices from three mice per group). c Representative 3D immunostaining images of CXCR4 (red) and glutamate (green) in the ACC of normal mice treated with ACSF or CXCL12. Scale bars, 20 μm. d Summarized data showing the signal intensity of CXCR4 in mice, as indicated in (c) (n = 8 slices from three mice per group). Data are shown as means ± SEM. ***P < 0.001. A two-tailed unpaired Student’s t test was used for (b, d)
Discussion
This study identifies a mechanism underlying microglia-meditated hyperactivity of ACCGlu neurons via CXCL12-CXCR4 signaling under diabetic conditions through which pain is generated. Central to this process, microglia are activated during the period of diabetic development, leading to an increase in microglial release of CXCL12 (Figs. 1f–l, 4d, e). CXCL12 acts on ACCGlu neuronal CXCR4, producing hyperactivity of ACCGlu neurons and thus promoting the development of diabetic pain symptoms.
Transformation of microglia to reactive states in response to pathology has been established for decades with highly mobile microglial processes and arborizations [40, 55]. These dynamic changes in microglia number and activation status, especially in the spinal and supraspinal central nervous system, have been implicated in multiple animal models for pain sensitization, such as in inflammation and neural injury that contributes to the development of chronic pain [56–58]. Different diabetic complications, e.g., neuropathic pain, require distinct peripheral and central sensitization mechanisms. Given that microglial activation results in multiple responses in the central nervous system, including changes in neural activity and tissue inflammation, it is reasonable to speculate that diabetes may exert long-term deleterious impacts through microglia. Our study showed that STZ-induced diabetes-like symptoms result in significant activation of ACC microglia, with associated changes in cellular morphology, numbers, and expression of inflammatory markers (Fig. 1f–l). Interestingly, these alterations did not occur until the seventh week after STZ treatment, at which point pain sensitization began to emerge (Figs. 1b and S2). The alleviation of pain sensitization through administration of microglia inhibitor starting from the fifth week after STZ treatment strongly indicates a correlation between microglial activation and diabetic pain symptoms (Fig. 2). Therefore, microglial activation in the ACC follows a similar time course to that of pain sensitization.
Compared to low glucose (10 mM), treatment with high glucose (35 mM) can activate microglia in a time-dependent manner in primary cultured rat microglia [59, 60], suggesting that the activation of microglia may be closely related to glucose concentration and time of glucose action. Moreover, the duration of diabetes progression or high hyperglycemia is apparently important for the emergence of pain phenotypes, as the mice displayed pain sensitization behavior at the seventh week after STZ injection. Glucose is known to form a concentration gradient between the circulating blood and the brain [61]. Moreover, glucose transport is tightly controlled at the blood-brain barrier and at the plasma membrane of neurons and glial cells [62], and diabetes leads to adverse effects on vasculature through microvascular injury, leading to pathogenesis of several cardiovascular diseases [63]. Therefore, in the early stage of diabetes, the blood glucose concentrations are not sufficiently high to activate microglia in the brain, whereas long-term hyperglycemia can affect blood-brain barrier integrity, oxidative stress in CNS microcapillaries [64], or cell metabolic dysfunction [65, 66], potentially leading to microglial activation and pain sensitization at the seventh week post-STZ injection. Behavioral outcomes of pain sensitization may be attributable to either dynamic network activity during progressive pain resulting from regional adaptations or the progression of a disease that promotes a maladaptive reactive microglial state.
The functional output of microglial cells has been proposed to occur via neuronal activity [67]. Numerous studies have shown that microglial proliferation and microglia-dependent synaptic plasticity respond to multiple behavioral consequences, including pain [68–71]. Our study showed that diabetic mice exhibit enhanced ACCGlu neuronal activity, accompanied by the occurrence of microglial activation and pain sensitization (Figs. 1 and 3a–i). Supporting this finding, inhibition of ACCGlu neuronal activity has been found to prevent the progression of pain sensitization during diabetes (Fig. 3j–l). Importantly, inactivation of microglia rescued the increase in STZ-induced ACCGlu neuronal activity and pain sensitization (Figs. 2, 4a, b). These results suggest a sufficient and necessary role of enhanced ACCGlu neuronal activity in the development of diabetic pain, which may be primed by microglial activation.
The molecular basis for microglia-mediated neuronal activity is still being uncovered, especially under different pathological circumstances. This complex process involves many molecules, including classic complement cascade-dependent phagocytic signaling, chemokine signaling, transforming growth factor β, and BDNF [13, 72–75]. Growing evidence supports that chemokines and their receptors play a role in inducing and maintaining pain or pain-related emotion. Previous studies have shown that CXCL13/CXCR5 signaling in the ACC is involved in neuropathic pain-related aversion via synaptic potentiation [76]. Our study demonstrated that the chemokine CXCL12 is remarkably upregulated in ACC microglia in mice with diabetic pain, whereas the expression of ACCGlu neuronal CXCR4 is decreased (Figs. 4d, 5e). Other studies have reported that β-arrestin is recruited following CXCR4 activation, priming CXCR4 internalization by facilitating clathrin and adaptin recruitment to the cell membrane [77], and ultimately attenuating CXCR4 levels on the membrane. This is consistent with previous reports showed that CXCR4 is internalized following stimulation with CXCL12 and is subsequently degraded, resulting in down-regulation of CXCR4 expression on the cell membrane [78]. Notably, pharmacological inhibition of CXCR4 reverses STZ-induced ACCGlu neuronal activity and pain sensitization without influencing microglial status (Figs. 5h–j and S8b, c). These results raise the possibility that microglial activation promotes the release of CXCL12, which subsequently acts on neuronal CXCR4, indicated by its decreased expression on ACCGlu neurons, and leads to hyperactivity in these neurons. This process is required for the development of diabetic pain, which is consistent with previous studies showed that CXCL12 expression was upregulated in the spinal cord and dorsal root ganglia in a rat model of posttraumatic neuropathic pain [79]. This hypothesis is also supported by our results that ACC infusion with CXCL12 produced an increase in hyperactivity of ACCGlu neurons and pain sensitization in normal mice.
CXCL12 can activate a series of downstream signaling pathways by binding to its receptor CXCR4 [80]; e.g., G-protein-mediated signaling pathways such as PI3K, MAPK and NF-κB that induce the release of intracellular Ca2+ also lead to neuronal hyperactivity. The neuronal hyperactivity and decreased expression of CXCR4 may be two independent pathways and intracellular events, both of which are triggered by CXCL12 action. Changes in synaptic plasticity can influence neuronal activity in the ACC [81]. CXCL12-CXCR4 chemokine signaling plays a critical role in modulating various nervous system developmental processes as well as the regulation of synaptic plasticity [80]. In mice with diabetes-associated pain, the activation of CXCR4 is largely responsible for ACCGlu neuron excitability. Long-term CXCR4 activation may lead to neuronal maladaptation and the modification of intrinsic neuronal excitability. After intrinsic excitability is established in ACCGlu neurons, these neurons remain in a persistent state of hyperexcitability, even following CXCR4 downregulation by CXCL12, consequently leading to maintenance of pain-related behaviors. The mechanism underlying these changes in neuronal activity by CXCR4 internalization or degradation warrants further investigation. Of note, CXCR4 shows a complex and often complementary expression pattern in both the developing and adult central nervous systems, and multiple functions of the signaling system have been shown in a variety of brain structures [80]. These include multiple pain-related regions, such as the spinal cord. In addition, CXCR4 is also expressed in neurons and endothelial cells [82], which might be related to neuroinflammation and thus is likely involved in chronic pain [83]. Previous reports have shown that excitatory CXCR4-CXCL12 signaling in Nav1.8-positive DRG neurons is an essential component in the pathogenesis of mechanical allodynia and small-fiber degeneration in mice with painful diabetic neuropathy [46]. In the spinal cord of rats with bone cancer, CXCR4 interacts with CXCL12 expressed in astrocytes, inducing neuronal sensitization and glial activation, leading to pain [84]; the activation of ERK1/2 by CXCL12-CXCR4 signaling in the spinal cord of rats has also been shown to play a role in postsurgical pain development [85]. Similarly, CXCL12-CXCR4 signaling in the spinal cord of rats with spinal nerve ligation was also shown to induce pain sensitization [86]. These studies collectively suggest that the regulatory mechanisms responsible for CXCL12-CXCR4 signaling in the spinal cord likely differ from those in the ACC. Therefore, we cannot rule out the role of CXCR4 outside the ACC in the development of diabetic pain, such as with microglia in the spinal cord. In the current study, pharmacological manipulation of the CXCL12-CXCR4 system was limited to the ACC rather than administered systemically, which at a minimum indicates that CXCR4 in the ACC is involved in diabetes-associated pain.
Taken together, the current study illustrates a molecular and cellular basis for better understanding of how diabetic conditions alter microglial activation and thereby exert long-term effects on synaptic plasticity, ultimately leading to pain. In this regard, drugs targeting CXCL12/CXCR4 signaling may serve as a promising class of analgesics for diabetic pain, or for preventing its development.
Supplementary information
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants 32025017, 32121002, 81971264, and 32271176), CAS Project for Young Scientists in Basic Research (YSBR-013), and Natural Science Foundation of Anhui Province (KJ2020A0138).
Author contributions
ZHS and XJS designed the studies, conducted most of the experiments and data analysis, and wrote the draft manuscript. PC, CLY, YM, and YJ conducted the behavioral experiments and data analyses and wrote the text of the final manuscript. MYX, WW, HTW, and XZ conducted some of the molecular and behavioral experiments. WJT, and ZZ were involved in the overall design of the study and the revision of the final manuscript. ZZ and WJT were involved in the overall design of the project, individual experiments, data analysis, and the writing of the final manuscript.
Data availability
All data necessary to understand and assess the conclusions of this study are available in the main text or the supplementary materials. There are no restrictions on data availability in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Zi-hua Song, Xiang-jie Song
Contributor Information
Wei Wang, Email: hfww2001@ustc.edu.cn.
Zhi Zhang, Email: zhizhang@ustc.edu.cn.
Wen-juan Tao, Email: wjtao01@ahmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41401-022-01046-7.
References
- 1.Feldman EL, Nave KA, Jensen TS, Bennett DLH. New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron. 2017;93:1296–313. doi: 10.1016/j.neuron.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schreiber AK, Nones CF, Reis RC, Chichorro JG, Cunha JM. Diabetic neuropathic pain: physiopathology and treatment. World J Diabetes. 2015;6:432–44. doi: 10.4239/wjd.v6.i3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ransohoff RM, Perry VH. Microglial physiology: unique stimuli, specialized responses. Annu Rev Immunol. 2009;27:119–45. doi: 10.1146/annurev.immunol.021908.132528. [DOI] [PubMed] [Google Scholar]
- 4.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 5.Miron VE, Priller J. Investigating microglia in health and disease: challenges and opportunities. Trends Immunol. 2020;41:785–93. doi: 10.1016/j.it.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci. 2020;23:194–208. doi: 10.1038/s41593-019-0566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berta T, Park CK, Xu ZZ, Xie RG, Liu T, Lü N, et al. Extracellular caspase-6 drives murine inflammatory pain via microglial TNF-α secretion. J Clin Invest. 2014;124:1173–86. doi: 10.1172/JCI72230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan Z, Kuhn JA, Wang X, Colquitt B, Solorzano C, Vaman S, et al. Injured sensory neuron-derived CSF1 induces microglial proliferation and DAP12-dependent pain. Nat Neurosci. 2016;19:94–101. doi: 10.1038/nn.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–7. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Sakaba T, Neher E. Direct modulation of synaptic vesicle priming by GABA(B) receptor activation at a glutamatergic synapse. Nature. 2003;424:775–8. doi: 10.1038/nature01859. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 12.Cserép C, Pósfai B, Lénárt N, Fekete R, László ZI, Lele Z, et al. Microglia monitor and protect neuronal function through specialized somatic purinergic junctions. Science. 2020;367:528–37. doi: 10.1126/science.aax6752. [DOI] [PubMed] [Google Scholar]
- 13.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badimon A, Strasburger HJ, Ayata P, Chen X, Nair A, Ikegami A, et al. Negative feedback control of neuronal activity by microglia. Nature. 2020;586:417–23. doi: 10.1038/s41586-020-2777-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou W, Jin Y, Meng Q, Zhu X, Bai T, Tian Y, et al. A neural circuit for comorbid depressive symptoms in chronic pain. Nat Neurosci. 2019;22:1649–58. doi: 10.1038/s41593-019-0468-2. [DOI] [PubMed] [Google Scholar]
- 16.Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016;17:485–96. doi: 10.1038/nrn.2016.68. [DOI] [PubMed] [Google Scholar]
- 17.Gregory C, Biafra A, Benjamin FG, Dong W, Mark JS, Grégory S. An amygdalar neural ensemble that encodes the unpleasantness of pain. Science. 2019;363:276–81. doi: 10.1126/science.aap8586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Selvarajah D, Wilkinson ID, Maxwell M, Davies J, Sankar A, Boland E, et al. Magnetic resonance neuroimaging study of brain structural differences in diabetic peripheral neuropathy. Diabetes Care. 2014;37:1681–8. doi: 10.2337/dc13-2610. [DOI] [PubMed] [Google Scholar]
- 19.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–9. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 20.Fischer TZ, Waxman SG. Neuropathic pain in diabetes-evidence for a central mechanism. Nat Rev Neurol. 2010;6:462–6. doi: 10.1038/nrneurol.2010.90. [DOI] [PubMed] [Google Scholar]
- 21.Guo B, Chen J, Chen Q, Ren K, Feng D, Mao H, et al. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat Neurosci. 2019;22:1223–34. doi: 10.1038/s41593-019-0445-9. [DOI] [PubMed] [Google Scholar]
- 22.Meda KS, Patel T, Braz JM, Malik R, Turner ML, Seifikar H, et al. Microcircuit mechanisms through which mediodorsal thalamic input to anterior cingulate cortex exacerbates pain-related aversion. Neuron. 2019;102:944–59.e3. doi: 10.1016/j.neuron.2019.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, et al. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010;330:1400–4. doi: 10.1126/science.1191792. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Zhou LJ, Wang J, Li D, Ren WJ, Peng J, et al. TNF-alpha differentially regulates synaptic plasticity in the hippocampus and spinal cord by microglia-dependent mechanisms after peripheral nerve injury. J Neurosci. 2017;37:871–81. doi: 10.1523/JNEUROSCI.2235-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blom SM, Pfister JP, Santello M, Senn W, Nevian T. Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex. J Neurosci. 2014;34:5754–64. doi: 10.1523/JNEUROSCI.3667-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koga K, Descalzi G, Chen T, Ko HG, Lu J, Li S, et al. Coexistence of two forms of LTP in ACC provides a synaptic mechanism for the interactions between anxiety and chronic pain. Neuron. 2015;85:377–89. doi: 10.1016/j.neuron.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Segerdahl AR, Themistocleous AC, Fido D, Bennett DL, Tracey I. A brain-based pain facilitation mechanism contributes to painful diabetic polyneuropathy. Brain. 2018;141:357–64. doi: 10.1093/brain/awx337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang BC, Ding TY, Guo CY, Bai XH, Cao DL, Wu XB, et al. NFAT1 orchestrates spinal microglial transcription and promotes microglial proliferation via c-MYC contributing to nerve injury-induced neuropathic pain. Adv Sci (Weinh) 2022;9:e2201300. doi: 10.1002/advs.202201300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yi MH, Liu YU, Liu K, Chen T, Bosco DB, Zheng J, et al. Chemogenetic manipulation of microglia inhibits neuroinflammation and neuropathic pain in mice. Brain Behav Immun. 2021;92:78–89. doi: 10.1016/j.bbi.2020.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu X, Tang HD, Dong WY, Kang F, Liu A, Mao Y, et al. Distinct thalamocortical circuits underlie allodynia induced by tissue injury and by depression-like states. Nat Neurosci. 2021;24:542–53. doi: 10.1038/s41593-021-00811-x. [DOI] [PubMed] [Google Scholar]
- 31.Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, et al. Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron. 2009;63:27–39. doi: 10.1016/j.neuron.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z, Cai YQ, Zou F, Bie B, Pan ZZ. Epigenetic suppression of GAD65 expression mediates persistent pain. Nat Med. 2011;17:1448–55. doi: 10.1038/nm.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang J, Zhong G, Wu J, Chen H, Jia Y. SOX2 recruits KLF4 to regulate nasopharyngeal carcinoma proliferation via PI3K/AKT signaling. Oncogenesis. 2018;7:1–13.. doi: 10.1038/s41389-018-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cardin JA, Carlén M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–7. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu H, Liu L, Tian Y, Wang J, Li J, Zheng J, et al. A disinhibitory microcircuit mediates conditioned social fear in the prefrontal cortex. Neuron. 2019;102:668–82.e5. doi: 10.1016/j.neuron.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 36.Lodato S, Arlotta P. Generating neuronal diversity in the mammalian cerebral cortex. Annu Rev Cell Dev Biol. 2015;31:699–720. doi: 10.1146/annurev-cellbio-100814-125353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan TT, Gao W, Song ZH, Long DD, Cao P, Hu R, et al. Glutamatergic neurons and myeloid cells in the anterior cingulate cortex mediate secondary hyperalgesia in chronic joint inflammatory pain. Brain Behav Immun. 2022;101:62–77. doi: 10.1016/j.bbi.2021.12.021. [DOI] [PubMed] [Google Scholar]
- 38.Tsantoulas C, Lainez S, Wong S, Mehta I, Vilar B, McNaughton PA. Hyperpolarization-activated cyclic nucleotide-gated 2 (HCN2) ion channels drive pain in mouse models of diabetic neuropathy. Sci Transl Med. 2017;9:eaam6072. doi: 10.1126/scitranslmed.aam6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Furman BL. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70:5. 47. 1–5. 47. 20. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- 40.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 41.Feng X, Wu CY, Burton FH, Loh HH, Wei LN. beta-arrestin protects neurons by mediating endogenous opioid arrest of inflammatory microglia. Cell Death Differ. 2014;21:397–406. doi: 10.1038/cdd.2013.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Szalay G, Martinecz B, Lenart N, Kornyei Z, Orsolits B, Judak L, et al. Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nat Commun. 2016;7:11499. doi: 10.1038/ncomms11499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H, et al. Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell. 2020;182:388–403.e15. doi: 10.1016/j.cell.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ling L, Xu H, Wang J, Li J, Tian Y, Zheng J, et al. Cell type–differential modulation of prefrontal cortical GABAergic interneurons on low gamma rhythm and social interaction. Sci Adv. 2020;6:eaay4073. doi: 10.1126/sciadv.aay4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu B, Jiang S, Li M, Xiong X, Zhu M, Li D, et al. Proteome-wide analysis of USP14 substrates revealed its role in hepatosteatosis via stabilization of FASN. Nat Commun. 2018;9:4770. doi: 10.1038/s41467-018-07185-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jayaraj ND, Bhattacharyya BJ, Belmadani AA, Ren D, Rathwell CA, Hackelberg S, et al. Reducing CXCR4-mediated nociceptor hyperexcitability reverses painful diabetic neuropathy. J Clin Invest. 2018;128:2205–25. doi: 10.1172/JCI92117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schiraldi M, Raucci A, Muñoz LM, Livoti E, Celona B, Venereau E, et al. HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med. 2012;209:551–63. doi: 10.1084/jem.20111739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mines MA, Goodwin JS, Limbird LE, Cui FF, Fan GH. Deubiquitination of CXCR4 by USP14 is critical for both CXCL12-induced CXCR4 degradation and chemotaxis but not ERK ativation. J Biol Chem. 2009;284:5742–52. doi: 10.1074/jbc.M808507200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bai L, Wang X, Li Z, Kong C, Zhao Y, Qian JL, et al. Upregulation of chemokine CXCL12 in the dorsal root ganglia and spinal cord contributes to the development and maintenance of neuropathic pain following spared nerve injury in rats. Neurosci Bull. 2016;32:27–40. doi: 10.1007/s12264-015-0007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang CH, Chuang JY, Fong YC, Maa MC, Way TD, Hung CH. Bone-derived SDF-1 stimulates IL-6 release via CXCR4, ERK and NF-κB pathways and promotes osteoclastogenesis in human oral cancer cells. Carcinogenesis. 2008;29:1483–92. doi: 10.1093/carcin/bgn045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang CY, Lee CY, Chen MY, Yang WH, Chen YH, Chang CH, et al. Stromal cell-derived factor-1/CXCR4 enhanced motility of human osteosarcoma cells involves MEK1/2, ERK and NF-kappaB-dependent pathways. J Cell Physiol. 2009;221:204–12. doi: 10.1002/jcp.21846. [DOI] [PubMed] [Google Scholar]
- 52.Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171–9. doi: 10.1016/j.semcancer.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 53.von Hundelshausen P, Agten SM, Eckardt V, Blanchet X, Schmitt MM, Ippel H, et al. Chemokine interactome mapping enables tailored intervention in acute and chronic inflammation. Sci Transl Med. 2017;9:eaah6650. doi: 10.1126/scitranslmed.aah6650. [DOI] [PubMed] [Google Scholar]
- 54.Bonham LW, Karch CM, Fan CC, Tan C, Geier EG, Wang Y, et al. CXCR4 involvement in neurodegenerative diseases. Transl Psychiatry. 2018;8:73. doi: 10.1038/s41398-017-0049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–27. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 56.Chen G, Zhang YQ, Qadri YJ, Serhan CN, Ji RR. Microglia in pain: detrimental and protective roles in pathogenesis and resolution of pain. Neuron. 2018;100:1292–311. doi: 10.1016/j.neuron.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao H, Alam A, Chen Q, Eusman MA, Pal A, Eguchi S, et al. The role of microglia in the pathobiology of neuropathic pain development: what do we know? Br J Anaesth. 2017;118:504–16. doi: 10.1093/bja/aex006. [DOI] [PubMed] [Google Scholar]
- 58.Peng J, Gu N, Zhou L, Eyo UB, Murugan M, Gan WB, et al. Microglia and monocytes synergistically promote the transition from acute to chronic pain after nerve injury. Nat Commun. 2016;7:12029. doi: 10.1038/ncomms12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Quan Y, Du J, Wang X. High glucose stimulates GRO secretion from rat microglia via ROS, PKC, and NF-kappaB pathways. J Neurosci Res. 2007;85:3150–9. doi: 10.1002/jnr.21421. [DOI] [PubMed] [Google Scholar]
- 60.Quan Y, Jiang CT, Xue B, Zhu SG, Wang X. High glucose stimulates TNFα and MCP-1 expression in rat microglia via ROS and NF-κB pathways. Acta Pharmacol Sin. 2011;32:188–93. doi: 10.1038/aps.2010.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Patching SG. Glucose transporters at the blood-brain barrier: function, regulation and gateways for drug delivery. Mol Neurobiol. 2017;54:1046–77. doi: 10.1007/s12035-015-9672-6. [DOI] [PubMed] [Google Scholar]
- 62.Koepsell H. Glucose transporters in brain in health and disease. Pflug Arch. 2020;472:1299–343. doi: 10.1007/s00424-020-02441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bogush M, Heldt NA, Persidsky Y. Blood brain barrier injury in diabetes: unrecognized effects on brain and cognition. J Neuroimmune Pharmacol. 2017;12:593–601. doi: 10.1007/s11481-017-9752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J Pharmacovigil. 2014;2:125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li W, Roy Choudhury G, Winters A, Prah J, Lin W, Liu R, et al. Hyperglycemia alters astrocyte metabolism and inhibits astrocyte proliferation. Aging Dis. 2018;9:674–84. doi: 10.14336/AD.2017.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brereton MF, Rohm M, Shimomura K, Holland C, Tornovsky-Babeay S, Dadon D, et al. Hyperglycaemia induces metabolic dysfunction and glycogen accumulation in pancreatic β-cells. Nat Commun. 2016;7:13496. doi: 10.1038/ncomms13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xanthos DN, Sandkuhler J. Neurogenic neuroinflammation: inflammatory CNS reactions in response to neuronal activity. Nat Rev Neurosci. 2014;15:43–53. doi: 10.1038/nrn3617. [DOI] [PubMed] [Google Scholar]
- 68.Luo C, Kuner T, Kuner R. Synaptic plasticity in pathological pain. Trends Neurosci. 2014;37:343–55. doi: 10.1016/j.tins.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 69.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 70.Gu N, Peng J, Murugan M, Wang X, Eyo UB, Sun D, et al. Spinal microgliosis due to resident microglial proliferation is required for pain hypersensitivity after peripheral nerve injury. Cell Rep. 2016;16:605–14. doi: 10.1016/j.celrep.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhou LJ, Peng J, Xu YN, Zeng WJ, Zhang J, Wei X, et al. Microglia are indispensable for synaptic plasticity in the spinal dorsal horn and chronic pain. Cell Rep. 2019;27:3844–859.e6. doi: 10.1016/j.celrep.2019.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vukojicic A, Delestrée N, Fletcher EV, Pagiazitis JG, Sankaranarayanan S, Yednock TA, et al. The classical complement pathway mediates microglia-dependent remodeling of spinal motor circuits during development and in SMA. Cell Rep. 2019;29:3087–100.e7. doi: 10.1016/j.celrep.2019.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Battista D, Ferrari CC, Gage FH, Pitossi FJ. Neurogenic niche modulation by activated microglia: transforming growth factor β increases neurogenesis in the adult dentate gyrus. Eur J Neurosci. 2006;23:83–93. doi: 10.1111/j.1460-9568.2005.04539.x. [DOI] [PubMed] [Google Scholar]
- 75.Coull JA, Beggs S, Boudreau D, Boivin D, Tsuda M, Inoue K, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 76.Wu XB, He LN, Jiang BC, Wang X, Lu Y, Gao YJ. Increased CXCL13 and CXCR5 in anterior cingulate cortex contributes to neuropathic pain-related conditioned place aversion. Neurosci Bull. 2019;35:613–23. doi: 10.1007/s12264-019-00377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thelen M. Dancing to the tune of chemokines. Nat Immunol. 2001;2:129–34. doi: 10.1038/84224. [DOI] [PubMed] [Google Scholar]
- 78.Janssens R, Struyf S, Proost P. The unique structural and functional features of CXCL12. Cell Mol Immunol. 2018;15:299–311. doi: 10.1038/cmi.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knerlich-Lukoschus F, von der Ropp-Brenner B, Lucius R, Mehdorn HM, Held-Feindt J. Spatiotemporal CCR1, CCL3(MIP-1α), CXCR4, CXCL12(SDF-1α) expression patterns in a rat spinal cord injury model of posttraumatic neuropathic pain. J Neurosurg Spine. 2011;14:583–97. doi: 10.3171/2010.12.SPINE10480. [DOI] [PubMed] [Google Scholar]
- 80.Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–31. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, et al. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005;25:11107–16. doi: 10.1523/JNEUROSCI.1678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bianchi ME, Mezzapelle R. The chemokine receptor CXCR4 in cell proliferation and tissue regeneration. Front Immunol. 2020;11:2109. doi: 10.3389/fimmu.2020.02109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meng YM, Liang J, Wu C, Xu J, Zeng DN, Yu XJ, et al. Monocytes/Macrophages promote vascular CXCR4 expression via the ERK pathway in hepatocellular carcinoma. Oncoimmunology. 2018;7:e1408745. doi: 10.1080/2162402X.2017.1408745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen W, Hu XM, Liu YN, Han Y, Chen LP, Wang CC, et al. CXCL12 in astrocytes contributes to bone cancer pain through CXCR4-mediated neuronal sensitization and glial activation in rat spinal cord. J Neuroinflammation. 2014;11:75. doi: 10.1186/1742-2094-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xing F, Kong C, Bai L, Qian J, Yuan J, Li Z, et al. CXCL12/CXCR4 signaling mediated ERK1/2 activation in spinal cord contributes to the pathogenesis of postsurgical pain in rats. Mol Pain. 2017;13:1744806917718753. doi: 10.1177/1744806917718753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu ZY, Song ZW, Guo SW, He JS, Wang SY, Zhu JG, et al. CXCL12/CXCR4 signaling contributes to neuropathic pain via central sensitization mechanisms in a rat spinal nerve ligation model. CNS Neurosci Ther. 2019;25:922–36. doi: 10.1111/cns.13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data necessary to understand and assess the conclusions of this study are available in the main text or the supplementary materials. There are no restrictions on data availability in the manuscript.