Abstract
Objective
To report a case of monozygotic dichorionic (DC) twins after a single cryopreserved blastocyst embryo transfer followed by genetic determination of zygosity postpartum.
Design
Case report.
Setting
University hospital.
Patients
A 26-year-old woman with polycystic ovary syndrome and her 36-year-old male partner with severe oligozoospermia, resulting in a 1.5-year history of primary infertility.
Interventions
Controlled ovarian stimulation and intracytoplasmic sperm injection treatment with single cryopreserved embryo transfer at blastocyst stage.
Main Outcome Measures
Ultrasound images of the fetuses and short tandem repeat genotyping postpartum.
Results
A DC twin pregnancy following a single cryopreserved blastocyst embryo transfer was confirmed at the first trimester screening. Confirmatory testing performed postpartum included short tandem repeat analysis determining monozygosity and pathology examination reporting DC placental configuration.
Conclusions
Dichorionic monozygotic twins are thought to arise from the splitting of an embryo before the blastocyst stage. This case suggests that placental configuration of monozygotic twins may not strictly depend on timing of embryo division. Genetic analysis is the only tool to confirm the zygosity.
Key Words: ICSI, single blastocyst embryo transfer, monozygotic dichorionic twins, DNA analysis
The exact mechanism leading to monozygotic twinning phenomena remains unclear. The universally accepted theory presented by Corner (1) in 1955 about the monozygotic-twin (MT) pregnancy development states that the chorionicity of MT depends on the timing of embryonic division as follows: dichorionic (DC) diamniotic (DA) MTs arise within 3 days postfertilization, monochorionic DA MTs occur between days 4 and 8, and monochorionic monoamniotic MTs occur after day 8 postfertilization.
Recent reports of monozygotic DC DA twins after a single embryo transfer (SET) at the blastocyst stage are contradictory to Corner’s (1) theory, as embryonic division of monozygotic DC twins is supposed to occur before blastocyst formation (2, 3, 4, 5, 6, 7, 8).
In this report, we describe another case of monozygotic DC DA twins after a single blastocyst embryo transfer. Given the limited genetic evidence in the literature, a genetic analysis was performed postpartum, confirming that the twins developed from the same zygote. This highlights the need to question the theory of MT development.
Case report
A couple with a 1.5-year history of primary infertility was referred to our unit for consultation. A 26-year-old woman with a history of oligomenorrhea was diagnosed with polycystic ovary syndrome according to the European Society of Human Reproduction and Embriology and American Society for Reproductive Medicine 2003 criteria (9). A 36-year-old partner’s semen analysis revealed severe oligozoospermia in accordance with the World Health Organization criteria (10). The couple was scheduled for intracytoplasmic sperm injection (ICSI) treatment.
An antagonist-based protocol was started, with the use of follitropin beta (150 IU) for 10 days. To avoid ovarian hyperstimulation syndrome, the final oocyte maturation was induced with gonadotropin-releasing hormone agonist, and the sonography-guided oocyte pick-up was performed 36 hours later. A total of 23 oocytes were retrieved and fertilized with the use of ICSI, yielding 10 blastocysts. All were vitrified due to the risk of ovarian hyperstimulation syndrome. No preimplantation genetic testing for aneuploidy was performed.
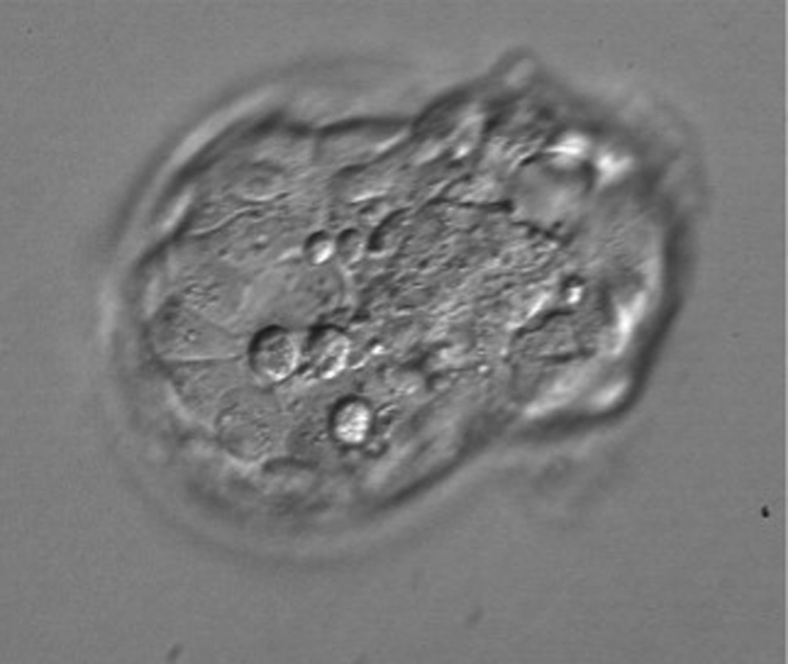
Three months later, a down-regulated frozen embryo transfer cycle was performed. Gonadotropin-releasing hormone agonist down-regulation combined with hormone replacement therapy was used. Suppression of ovulation with absence of dominant follicle in the ovaries was confirmed with ultrasound imaging. On the morning of transfer, a high-quality blastocyst (grade 4AA according to the Gardner morphological criteria) was thawed (11). No assisted hatching was performed before transfer. Two hours after warming, the blastocyst started to hatch (Fig. 1). Transfer was performed with the use of transabdominal ultrasound guidance.
Figure 1.
Blastocyst hatching just before the embryo transfer.
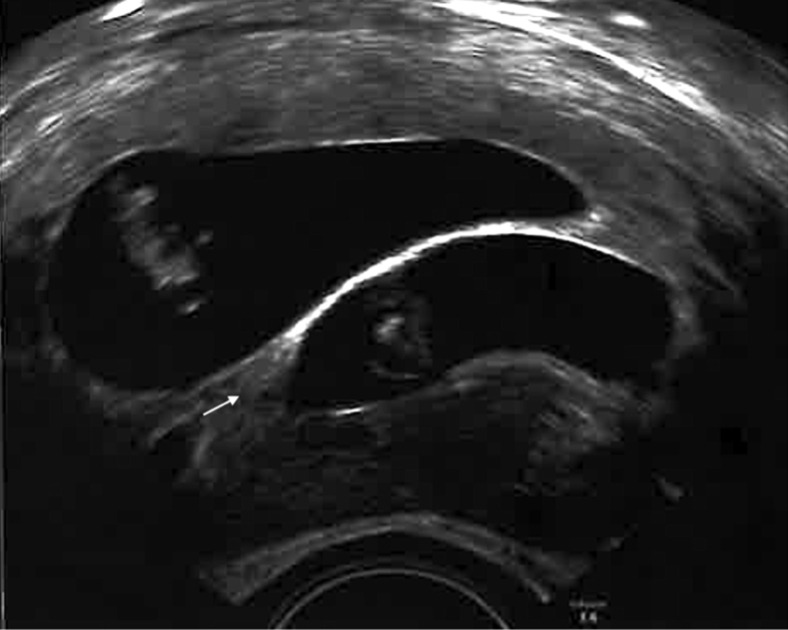
Fifteen days later, serum ß-human chorionic gonadotropin was found to be positive (2287 mU/mL), and at week 7 of gestation, an ultrasound examination revealed two fetuses with clearly separated chorionic cavities. According to International Society of Ultrasound in Obstetrics and Gynecology’s recommendation (12), chorionicity was confirmed, demonstrating the typical lambda (λ) sign (Fig. 2).
Figure 2.
The gestational ultrasound image suggesting dichorionic twin pregnancy with typical lambda (λ) sign (arrow).
At week 25 of gestation, antenatal corticosteroid therapy for fetal maturation was administered due to cervix insufficiency, followed by a successful placement of cervical pessary. At 31 + 1 weeks of gestation, the patient had a preterm premature rupture of membranes with preterm contractions and delivered two healthy male infants via C-section, weighing 1450 g and 1830 g, respectively.
The postpartum pathology examination results revealed DC placental configuration with fusion of both placentas.
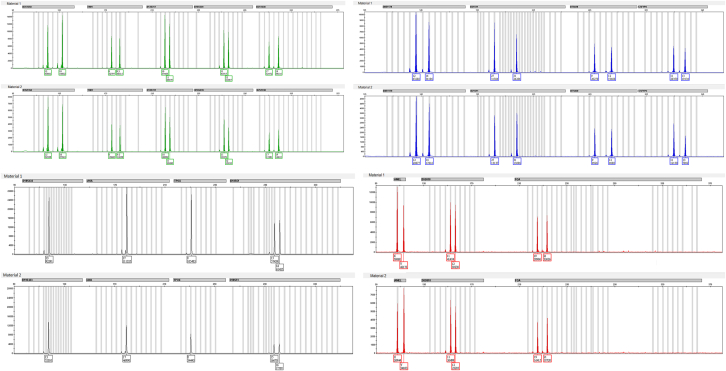
To confirm the zygosity of the twins, short tandem repeat (STR) genotyping was performed using the AmpFℓSTR Identifiler Plus Polymerase Chain Reaction AmplificationKit (Applied Biosystems, Waltham, MA). The kit simultaneously amplified 15 STR loci and an amelogenin gender-determining marker. Genotypes of the male infants were identical at all marker loci, confirming their monozygosity (Fig. 3).
Figure 3.
Short tandem repeat profile determining the monozygosity. Genotypes of twins were identical at all informative marker loci.
The patient gave consent for this case report to be published.
Discussion
Multiple gestations after assisted reproductive technologies (ART) are mostly dizygotic because of simultaneous transfer of multiple embryos. Elective SET has been the most effective policy to reduce the prevalence of ART-related multiple pregnancies (13). However, the incidence of MTs after fertility treatment has been reported to be significantly higher compared with natural conceptions (14).
The exact overall incidence of MT remains unclear. The rate after spontaneous conception was estimated by assessing live birth data rather than early ultrasound findings, as in most pregnancies after ART (13). Most studies investigating the MT rate after ART defined monozygosity as two fetuses within one gestational sac or the number of fetuses being higher than the number of transferred embryos. This excludes the monozygotic DC twins and misses the MT after multiple embryo transfers (15). Only genetic testing could reliably detect the true MT incidence.
The prevalence of MT in the context of ART has been widely debated, and several contributing risk factors have been proposed. The manipulation of the zona pellucida when performing ICSI, assisted hatching, and embryo biopsy has been reported to augment the frequency of MT pregnancies. Furthermore, stimulation protocols, changes in culture media, and maternal age were hypothesized as risk factors (16). Recent studies have failed to confirm such associations (14, 15, 16, 17, 18). The most consistently reported risk factor for monozygotic twinning after ART is a blastocyst transfer (17). It has been speculated that prolonged exposure to culture media as well as increased sensitivity of blastocyst embryos to manipulation in the laboratory may lead to a higher rate of MT (16). Papanikolaou et al. (19) assessed the incidence of MT on the basis of only SET cycles. This eliminated some bias of MT incidence calculations discussed above. In their study, they confirmed a higher frequency of MT after ART. Interestingly, they found no association between the probability of MT and blastocyst transfer compared with cleavage-stage transfer.
In our case, the DC twin pregnancy after a single blastocyst transfer after the ICSI treatment was confirmed at the first trimester screening. The origin of DC twin pregnancies can be monozygotic or dizygotic. Embryo division of monozygotic DC twins is supposed to occur before the blastocyst formation, before the development of the trophectoderm and inner cell mass (1). According to this theory, the twins could only be dizygotic because of concomitant spontaneous conception. However, we performed a down-regulated frozen embryo transfer cycle with suppression of ovulation and there was an absence of a dominant follicle in the ovaries, which was confirmed with ultrasound monitoring, meaning that no concomitant conception could occur. Postpartum genetic analysis confirmed that the twins were indeed monozygotic.
Other monozygotic DC DA twin pregnancies after single blastocyst embryo transfer have been reported (2, 3, 4, 5, 6, 7, 8). Although most of these reports lack genetic confirmation, the existing data and our report suggest that Corner’s (1) development model, which is strictly on the basis of timing of zygotal or embryonic splitting, should be reconsidered, at least when referring to the ART setting.
In addition, the laboratory records do not support the proposed theory either. Assisted reproductive technologies allows direct observation of an early embryonic development in vitro. Knopman et al. (4) and Kyono (3) reported that in the laboratory work of 30 and 15years, respectively, no spontaneous division of an embryo before reaching the blastocyst stage has been observed. Moreover, Van Langendonckt et al. (20) and Behr and Milki (21) described a blastocyst divided in vitro into two separate blastocysts, both with visible trophectoderm and inner cell mass.
Thus, alternatives to Corner’s (1) model have been proposed. Dirican (22) hypothesized that monozygotic monochorionic and monozygotic DC twins all develop from a blastocyst with two inner cell masses, and chorionicity determination occurs in utero depending on events during the implantation. Herranz (23) offers a developmental model where all MTs develop from two twin fertilizable cells originating from the first zygotic division, whereas chorionicity depends on the fusion of membranes. Furthermore, mechanisms disrupting the oocyte polarity have been debated to cause the MT (24).
The data on monozygotic DC twins after a single blastocyst embryo transfer are scarce. Zygosity, on the basis of only ultrasound findings, can easily be misdiagnosed: same-sex DC twins after a SET can be misinterpreted as dizygotic, which probably leads to cases like ours being underreported (6). We present a rare report confirming the zygosity of monozygotic DC twins after a single blastocyst embryo transfer using DNA analysis, showing that a single embryo can split into DC twins after the blastocyst has been formed, at least in the context of ART.
Unfortunately, no time-lapse embryo examination was used to detect potential signs of division precisely.
Conclusion
Dichorionic MTs are thought to arise from the splitting of an embryo before the blastocyst stage. This case suggests that placental configuration of MTs may not strictly depend on timing of embryo division. Considering the limited literature, case reports are important and highlight the need for studies that can further unravel the complexity of monozygotic twinning and help to reduce the risk of multifetal gestations after ART.
Footnotes
N.S. has nothing to disclose. M.B. has nothing to disclose. S.F. has nothing to disclose. R.H. has nothing to disclose. I.O. has nothing to disclose. H.F. has nothing to disclose. P.K. has nothing to disclose. I.S. has nothing to disclose. M.K. has nothing to disclose.
References
- 1.Corner G.W. The observed embryology of human single-ovum twins and other multiple births. Am J Obstet Gynecol. 1955;70:933–951. doi: 10.1016/0002-9378(55)90001-6. [DOI] [PubMed] [Google Scholar]
- 2.Konno H., Murakoshi T., Miura K., Masuzaki H. The incidence of dichorionic diamniotic twin pregnancy after single blastocyst embryo transfer and zygosity: 8 years of single-center experience. Twin Res Hum Genet. 2020;23:51–54. doi: 10.1017/thg.2020.5. [DOI] [PubMed] [Google Scholar]
- 3.Kyono K. The precise timing of embryo splitting for monozygotic dichorionic diamniotic twins: when does embryo splitting for monozygotic dichorionic diamniotic twins occur? Evidence for splitting at the morula/blastocyst stage from studies of in vitro fertilization. Twin Res Hum Genet. 2013;16:827–832. doi: 10.1017/thg.2013.32. [DOI] [PubMed] [Google Scholar]
- 4.Knopman J.M., Krey L.C., Oh C., Lee J., McCaffrey C., Noyes N. What makes them split? Identifying risk factors that lead to monozygotic twins after in vitro fertilization. Fertil Steril. 2014;102:82–89. doi: 10.1016/j.fertnstert.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 5.Li H., Shen T., Sun X. Monozygotic dichorionic-diamniotic pregnancies following single frozen-thawed blastocyst transfer: a retrospective case series. BMC Pregnancy Childbirth. 2020;20:768. doi: 10.1186/s12884-020-03450-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shibuya Y., Kyono K. A successful birth of healthy monozygotic dichorionic diamniotic (DD) twins of the same gender following a single vitrified-warmed blastocyst transfer. J Assist Reprod Genet. 2012;29:255–257. doi: 10.1007/s10815-011-9707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundaram V., Ribeiro S., Noel M. Multi-chorionic pregnancies following single embryo transfer at the blastocyst stage: a case series and review of the literature. J Assist Reprod Genet. 2018;35:2109–2117. doi: 10.1007/s10815-018-1329-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jundi S.I., Pereira N.C.A., Merighi T.M., Santos J.F.D., Yadid I.M., Coslovsky M., et al. Monozygotic dichorionic-diamniotic twin pregnancy after single embryo transfer at blastocyst stage: a case report. JBRA Assist Reprod. 2021;25:168–170. doi: 10.5935/1518-0557.20200052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. WHO laboratory manual for the examination and processing of human semen. 6th ed. WHO Press; Geneva: 2021. 00–0. [Google Scholar]
- 11.Gardner D.K., Lane M., Stevens J., Schlenker T., Schoolcraft W.B. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73:1155–1158. doi: 10.1016/s0015-0282(00)00518-5. [DOI] [PubMed] [Google Scholar]
- 12.Khalil A., Rodgers M., Baschat A., Bhide A., Gratacos E., Hecher K., et al. ISUOG practice guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol. 2016;47:247–263. doi: 10.1002/uog.15821. [DOI] [PubMed] [Google Scholar]
- 13.Vega M., Zaghi S., Buyuk E., Jindal S. Not all twins are monozygotic after elective single embryo transfer: analysis of 32,600 elective single embryo transfer cycles as reported to the Society for Assisted Reproductive Technology. Fertil Steril. 2018;109:118–122. doi: 10.1016/j.fertnstert.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 14.Vitthala S., Gelbaya T.A., Brison D.R., Fitzgerald C.T., Nardo L.G. The risk of monozygotic twins after assisted reproductive technology: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:45–55. doi: 10.1093/humupd/dmn045. [DOI] [PubMed] [Google Scholar]
- 15.Hviid K.V.R., Malchau S.S., Pinborg A., Nielsen H.S. Determinants of monozygotic twinning in ART: a systematic review and a meta-analysis. Hum Reprod Update. 2018;24:468–483. doi: 10.1093/humupd/dmy006. [DOI] [PubMed] [Google Scholar]
- 16.Busnelli A., Dallagiovanna C., Reschini M., Paffoni A., Fedele L., Somigliana E. Risk factors for monozygotic twinning after in vitro fertilization: a systematic review and meta-analysis. Fertil Steril. 2019;111:302–317. doi: 10.1016/j.fertnstert.2018.10.025. [DOI] [PubMed] [Google Scholar]
- 17.Ikemoto Y., Kuroda K., Ochiai A., Yamashita S., Ikuma S., Nojiri S., et al. Prevalence and risk factors of zygotic splitting after 937 848 single embryo transfer cycles. Hum Reprod. 2018;33:1984–1991. doi: 10.1093/humrep/dey294. [DOI] [PubMed] [Google Scholar]
- 18.Mateizel I., Santos-Ribeiro S., Done E., Van Landuyt L., Van de Velde H., Tournaye H., et al. Do ARTs affect the incidence of monozygotic twinning? Hum Reprod. 2016;31:2435–2441. doi: 10.1093/humrep/dew216. [DOI] [PubMed] [Google Scholar]
- 19.Papanikolaou E.G., Fatemi H., Venetis C., Donoso P., Kolibianakis E., Tournaye H., et al. Monozygotic twinning is not increased after single blastocyst transfer compared with single cleavage-stage embryo transfer. Fertil Steril. 2010;93:592–597. doi: 10.1016/j.fertnstert.2008.12.088. [DOI] [PubMed] [Google Scholar]
- 20.Van Langendonckt A., Wyns C., Godin P.A., Toussaint-Demylle D., Donnez J. Atypical hatching of a human blastocyst leading to monozygotic twinning: a case report. Fertil Steril. 2000;74:1047–1050. doi: 10.1016/s0015-0282(00)01554-5. [DOI] [PubMed] [Google Scholar]
- 21.Behr B., Milki A.A. Visualization of atypical hatching of a human blastocyst in vitro forming two identical embryos. Fertil Steril. 2003;80:1502–1503. doi: 10.1016/j.fertnstert.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Dirican E.K., Olgan S. On the origin of zygosity and chorionicity in twinning: evidence from human in vitro fertilization. J Assist Reprod Genet. 2021;38:2809–2816. doi: 10.1007/s10815-021-02294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herranz G. The timing of monozygotic twinning: a criticism of the common model. Zygote. 2015;23:27–40. doi: 10.1017/S0967199413000257. [DOI] [PubMed] [Google Scholar]
- 24.Scott L. The origin of monozygotic twinning. Reprod Biomed Online. 2002;5:276–284. doi: 10.1016/s1472-6483(10)61833-0. [DOI] [PubMed] [Google Scholar]