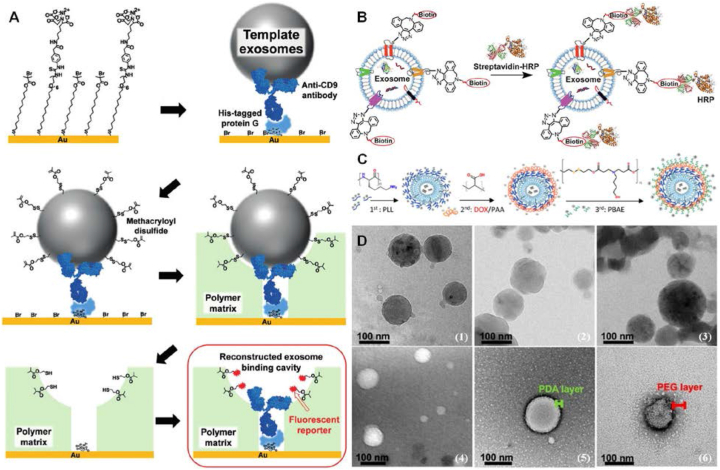
Figure 6.
Chemical engineering on the EXOs by different methodologies. (A) Disulfide bridging-modified template EXOs for detecting intact EXOs. Firstly, His-tagged protein G was anchored through NiII complex with nitrilotriacetic acid (NTA) groups on the gold substrate modified with a mixed self-assembled monolayer, by which anti-CD9 antibodies were immobilized by interaction with His-tagged protein G. The template EXOs were then specifically captured on the substrate through antigen/antibody binding and subsequently, their surface was modified with methacryloyl disulfide. The captured EXOs were imprinted by a polymer matrix on the substrate. After removal of the template EXOs, the disulfide linker would be transformed into a free thiol inside EXO-binding cavities, allowing the fluorescent reporter to be conjugated on the thiol groups. By monitoring the fluorescent signal inside cavities, intact EXOs could be sufficiently detected with high sensitivity. Referenced with permission from Wiley-VCH. (B) Biotinylation of EXOs for intracellular delivery. EXOs were firstly biotinylated with DBCO-PEG4-biotin by “click chemistry”. Afterward, the streptavidin-functionalized cargos could be specifically crosslinked onto biotin conjugated on EXOs. (C) Trlayered shell assembly of polyelectrolytes formed on the siRNA-loaded EXOs by layer-by-layer deposition serves as the carrier for DOX loading. 1st layer: cationic poly(l-lysine) (PLL); 2nd layer: anionic poly(acrylic acids) (PAA); 3rd layer: poly(b-amino esters) (PBAE). Referenced from American Chemical Society (Copyright 2020). (D) Morphology characterization of polymer-modified EXOs. Cryo-TEM images of (1) bare EXOs, (2) EXO@PDA, and (3) EXO@PDA@PEG; TEM images of (4) bare EXOs, (5) EXO@PDA, and (6) EXO@PDA@PEG2000. Referenced with permission from Wiley-VCH.