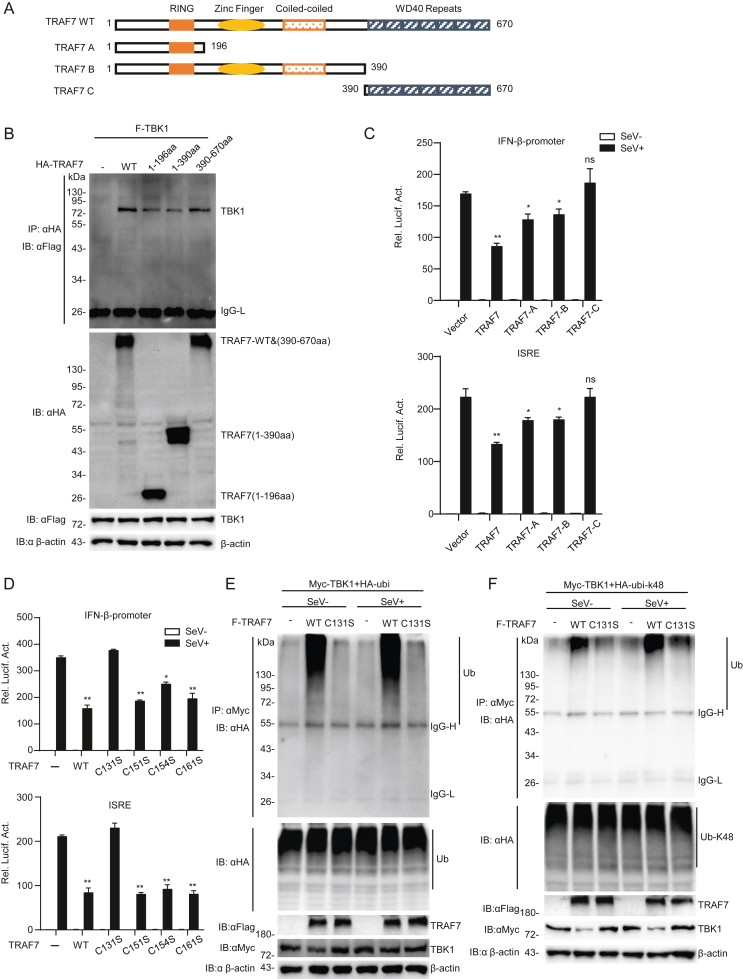
Fig. 6.
The RING domain is necessary for TRAF7 functions. A A schematic diagram of wild-type and truncated human TRAF7 mutants. B TRAF7 interacts with TBK1 through its RING domain and WD40 repeat domain. HEK 293 cells (2 × 105) were transfected with TBK1 and TRAF7 deletion mutants for 24 h and treated with MG132 before Co-IP and immunoblotting analysis. C TRAF7 relies on the RING domain to repress the activation of reporter genes. HEK 293 cells (2 × 105) were transfected with the indicated plasmids, and after 12 h, cells were treated or untreated with SeV (MOI = 1) for 12 h before luciferase analysis. D The conserved cysteine residues at position 131 of TRAF7 are necessary for TRAF7's RING domain functions. HEK 293 cells (2 × 105) were transfected with the indicated plasmids, and after 12 h, cells were treated or untreated with SeV (MOI = 1) for 12 h before luciferase analysis. E and F The conserved cysteine residues at position 131 of TRAF7 are necessary for its E3 ligase activity. HEK 293 cells (2 × 105) were transfected with the indicated plasmids; after 12 h, the cells were infected or uninfected with SeV (MOI = 1) for 10 h before Co-IP and immunoblotting analysis. (∗P < 0.05; ∗∗P < 0.01; ns, no significant difference).