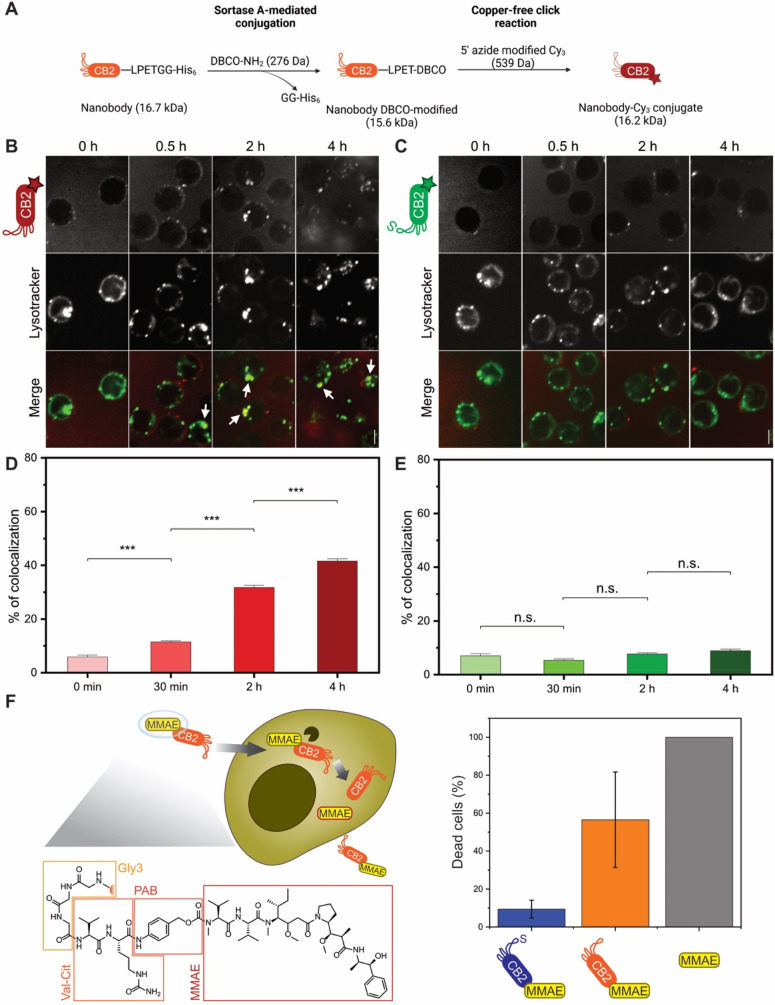
Figure 5.
CB2 can be internalized by B cell lymphoma and used for drug delivery. (A) Illustration of the conjugation approach. The C-terminus of CB2 is functionalized with a dibenzocyclooctyne (DBCO) group using the sortase reaction. Subsequently, Cy3-azide is attached to CB2-DBCO via copper-free click chemistry, yielding fluorescent CB2 linked to Cy3 via a triazole moiety. (B–E) Nanobody internalization assay. Confocal fluorescence microscopy images of live SC-1 cells incubated with 100 μg of (B) CB2–Cy3 or (C) C105SCB2–Cy3 for increasing time intervals (0 min, 30 min, 2 h, 4 h). Top, nanobody; bottom, lysotracker. Colors in merge: red = Cy3, green = lysotracker. White arrows indicate colocalization between nanobody and lysotracker. Scale bar = 5 μm. Quantification of colocalization in live-cell microscopy of lysotracker with (D) CB2–Cy3 or (E) C105SCB2–Cy3. Values represent mean ± SEM (n > 300 cells/time point from three independent experiments), (***) p < 0.001, (n.s.) p > 0.05. Note that colocalization with lysotracker significantly increases over time for CB2–Cy3, whereas C105SCB2–Cy3 does not accumulate in the lysosomes. (F) Drug delivery with internalizing CB2. Left panel: Proposed mechanism of action. MMAE is coupled to CB2 via a cleavable linker using srtA. Val–cit = valine–citrulline, PAB = para-aminobenzylcarbamate, MMAE = monomethyl auristatin E. MMAE is released inside the cell by cathepsin (black) cleaving between val–cit. Right panel: Cytotoxicity assay with SC-1 cells. Dead cells (%) after 48 h incubation with 10 nM CB2-MMAE, C105SCB2-MMAE, or MMAE. Note that only CB2-MMAE and MMAE alone show considerable cytotoxic activity. Cytotoxicity was measured using the CCK8 kit and normalized to 100% and 0% based on positive (TritonX) and negative (unconjugated CB2) controls, respectively. Values represent mean ± SEM (N = 3).