Fig. 7. Mef2d deficiency leads to enhanced SAP expression, IL-21 production and GC-Tfh differentiation of antigen-specific CD4 T cells.
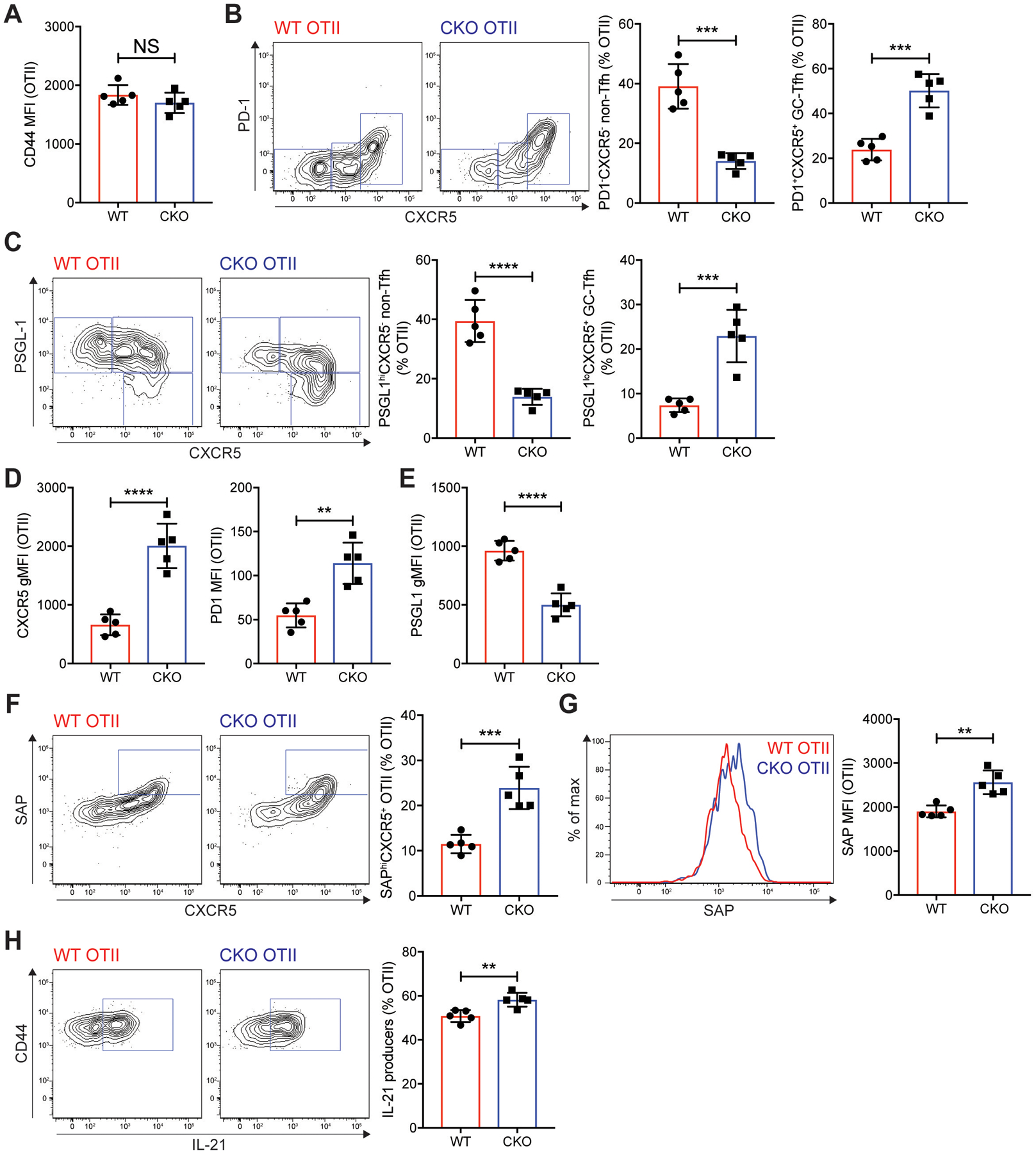
WT control and Mef2d CKO (Cd4CreMef2dfl/fl) CD45.2 OTII CD4 T cells were transferred into CD45.1 B6.SJL mice, which were immunized subcutaneously with NP-OVA. Seven days later, popLNs were examined for GC-Tfh differentiation, SAP expression, and IL-21 production.
(A) CD44 MFIs of the donor OTII CD4 T cells in the popLNs.
(B and C) Flow cytometry plots of the transferred OTII CD4 T cells. Gates indicate non-Tfh (PD-1−CXCR5− or PSGL-1hiCXCR5−), Tfh (PD-1−CXCR5+ or PSGL-1hiCXCR5+), and GC-Tfh (PD-1+CXCR5+ or PSGL-1loCXCR5+) cells. The frequencies of non-Tfh and GC-Tfh cells were calculated.
(D) CXCR5 gMFIs and PD-1 MFIs of the donor OTII CD4 T cells were quantified.
(E) PSGL-1 gMFIs of the donor cells were calculated.
(F and G) SAP expression of the WT control and Mef2d CKO OTII CD4 T cells were examined. Flow cytometry plots of the donor cells with gates indicating SAPhiCXCR5+ compartment (F). Overlaid SAP histograms (G). SAPhiCXCR5+ cell frequencies and intracellular SAP MFIs were quantified.
(H) Flow cytometry plots of the transferred OTII CD4 T cells. Gates indicate IL-21 producing cells. The frequencies of IL-21 producers were quantified.
Representative of two independent experiments with n=5 mice per group.
Error bars indicate mean with SD. Statistical significance values were determined using two-tailed Student’s t-test. NS, statistically non-significant; ** p <0.01; *** p <0.001; **** p <0.0001.
