Abstract
Purpose
To compare the performance of Neutrophil-to-Lymphocyte Ratio (NLR) with that of Platelet-to-Lymphocyte Ratio (PLR) in diagnosing neonatal sepsis (NS).
Methods
PubMed and Embase were searched for relevant studies from the inception of the databases to May, 2022. The pooled sensitivity (SEN), specificity (SPE), and area under the receiver operator characteristic curve (AUC) were measured.
Results
Thirteen studies involving 2610 participants were included. The SEN, SPE, and AUC of NLR were 0.76 (95%CI: 0.61–0.87), 0.82 (95%CI: 0.68–0.91), and 0.86 (95%CI: 0.83–0.89), respectively, and those of PLR were 0.82 (95%CI: 0.63–0.92), 0.80 (95%CI: 0.24–0.98), and 0.87 (95%CI: 0.83–0.89), respectively. Significant heterogeneity was observed among the studies. Subgroup analysis and meta-regression showed that types of sepsis (p = 0.01 for SEN), gold standard (p = 0.03 for SPE), and pre-set threshold (p<0.05 for SPE) might be the sources of heterogeneity for NLR, whereas the pre-set threshold (p<0.05 for SPE) might be the source of heterogeneity for PLR.
Conclusions
NLR and PLR would be of great accuracy for the diagnosis of NS, and the two indicators have similar diagnostic performance. However, the overall risk of bias was high, and significant heterogeneity was identified among the included studies. The results of this study should be interpreted prudently, and the normal or cut-off values and the type of sepsis should be considered. More prospective studies are needed to further support the clinical application of these findings.
Keywords: Neutrophil-To-Lymphocyte ratio, Platelet-To-Lymphocyte ratio, Neonatal sepsis, Diagnostic test, meta-analysis
Introduction
Sepsis refers to systemic inflammatory reaction syndrome caused by microbial infection, the pathogens include bacteria, viruses, fungus, and protozoon. Neonatal sepsis (NS) is a common cause of neonatal death [1–3]. It is of high morbidity especially in newborns, with approximately 3 million cases worldwide and a mortality rate ranging from 11–19% [4]. Sepsis falls into early-onset sepsis (EOS, Septicemia that occurs within 72 h after delivery) and late-onset sepsis (LOS, Septicemia that occurs more than 72 h after delivery) according to the time of onset [5]. The condition of infants with sepsis changes rapidly and the treatment remains intractable, leading to a high mortality rate. Early diagnosis and timely intervention are of great importance for improving the prognosis of NS newborns [6].
NS at an early stage often has atypical symptoms and signs. The current gold standard for NS diagnosis is blood culture, which requires a long culturing time with a low positive rate, making the early diagnosis extremely difficult [7]. There is an urgent need for a rapid biomarker and high specificity to help the early identification of NS before getting a positive blood culture. However, there is no excellent biomarker to be used in predicting NS. Numerous biomarkers have already been investigated for the early detection of sepsis. The classification of these markers includes risk prediction, diagnosis, monitoring, and outcome [8, 9]. Procalcitonin and CD14 are demonstrated to be effective markers, while the costs of detection are often unaffordable for low- and middle-income nations like Brazil [9, 10].
Studies have demonstrated that Neutrophil-To-Lymphocyte Ratio (NLR) and Platelet-To-Lymphocyte Ratio (PLR) could be applied as biomarkers for NS [11–13]. The normal ranges of NLR and PLR do not have been unified, which depends on the age and health status of the neonates. NLR and PLR present to be applicable, feasible, and affordable approaches for rapid diagnosis of NS, and are of great significance for the early diagnosis, treatment, and prevention of NS [14]. However, whether NLR or PLR is a better indicator for the diagnosis of neonatal sepsis and its diagnostic accuracy is still debated.
The aim of this study is to summarize the current evidence to evaluate the diagnostic performance of NLR and PLR for NS, and assess the sensitivity and specificity of NLR and PLR for NS diagnosis, so as to provide a reference for clinical early identification of NS.
Materials and methods
Search strategy
PubMed and Embase were searched, from inception to May, 2022, for potentially eligible studies using an algorithm based on combined words. Search items mainly included “Neonatal Sepsis”, “NLR”, “neutrophil to lymphocyte ratio”, “PLR”, and “platelet to lymphocyte ratio”. Taking PubMed for example, the search strategy was designed as follows: (NLR OR neutrophil to lymphocyte ratio OR neutrophil-lymphocyte ratio OR PLR OR platelet to lymphocyte ratio OR platelet-lymphocyte ratio)AND(Neonatal Sepsis OR Neonatal Sepsis OR Sepsis, Neonatal OR Neonatal Late-Onset Sepsis OR Late-Onset Sepsis, Neonatal OR Neonatal Late Onset Sepsis OR Neonatal Late-Onset Sepsis OR Sepsis, Neonatal Late-Onset OR Sepsis, Neonatal Late-Onset OR Neonatal Early-Onset Sepsis OR Early-Onset Sepsis, Neonatal OR Early-Onset Sepsis, Neonatal OR Neonatal Early Onset Sepsis OR Neonatal Early-Onset Sepsis OR Sepsis, Neonatal Early-Onset OR Sepsis, Neonatal Early-Onset). Reference lists of included studies were also searched for potentially eligible studies.
Inclusion and exclusion criteria
Studies meeting the following criteria were included: (a)Evaluating the diagnostic performance of NLR and PLR for NS; (b)Retrospective study, prospective study, or cross-sectional study. Articles will be excluded for the following reasons:(a)Case-report, literature review, conference summary, abstract unavailable, meta-analysis, letter, and comments;(b)Data unextractable;(c)Study reported and published in non-English.
Data extraction and quality assessment
Data extraction was conducted by two reviewers independently. Extracted data contained: name of the first author, characteristics of the study (publication date, nationality, study design, and gold standard), characteristics of participants, types of sepsis, samples for test, diagnostic cut-off value, true negative (TN), false negative (FN), true positive (TP), and false positive (FP). Any disagreement was settled via discussion between the reviewers.
Quality assessment was conducted by two reviewers independently using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2). Each included study was assessed according to the following domains: patient selection, index test, reference standard, and flow and timing. These domains were assessed according to the risk of bias, and the applicability was graded as “high”, “low”, or “unclear” [15]. Disagreements between the reviewers were settled through discussion.
Statistical analysis
All data analyses were performed using Stata 15.1 software. Pooled sensitivity (SEN) and specificity (SPE) were analyzed using a bivariate random-effect model. The receiver operator characteristic curve (ROC) was plotted and the area under the curve (AUC) was calculated. For an I2>50%, subgroup analysis and meta-regression were performed to identify the sources of heterogeneity, and the sources were classified based on the following aspects: sample size, nationality, study design, gold standard, types of sepsis, source of participants, and diagnostic cut-off value for sepsis) Deek’s funnel plot was provided to assess the publication bias. A p value less than 0.05 indicated significant publication bias.
Results
Literature search and study selection
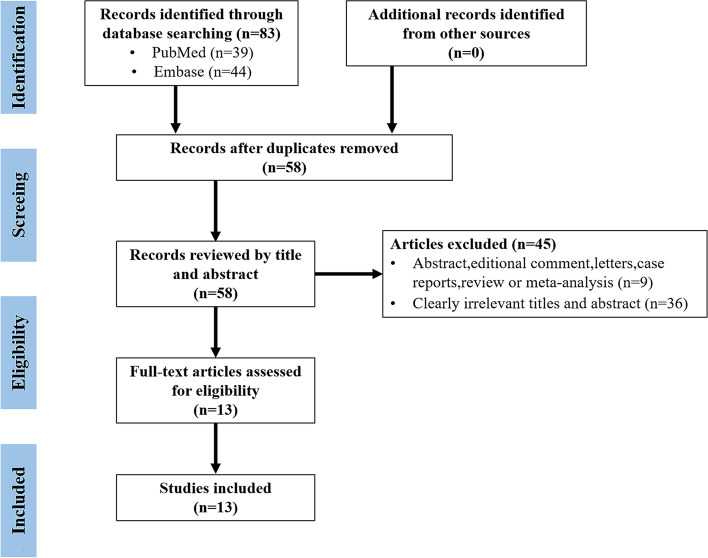
There were 83 articles retrieved (PubMed: 39, Embase: 44), as shown in Fig. 1, and 25 duplicates were removed. Among the remaining 58 articles, 45 were excluded after browsing titles and abstracts. Finally, 13 studies were included after reading the full texts [13, 14, 16–26].
Fig. 1.
the PRISMA flow diagram of study selection
Characteristics and quality assessment of included studies
Characteristics of 13 included studies containing 2610 participants (1862 for NLR, 159 for PLR, and 589 for both NLR and PLR) were summarized, as shown in Table 1. Among the 13 included studies, 5 were conducted in Turkey, 3 in China, 2 in Indonesia, 1 in India, 1 in Israel, and 1 in Egypt. As for study design, 6 were retrospective design, 5 were prospective, and 2 were cross-sectional. For the gold standard, 4 studies applied blood culture, 2 applied clinical diagnostic criteria, and 7 adopted both. For types of sepsis, 6 studies reported EOS, 2 reported LOS, and 5 reported both the EOS and LOS.
Table 1.
The characteristics of the included studies
| Author | Year | Country | Study design | Preterm and Term Infants | Gold standard | Type of Sepsis | Specimen | Center | Index | cut-off | N | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kurt, A et al. [16] | 2022 | Turkey | Retro | Term Infants(100%) | Clinical diagnosis, blood culture | EOS&LOS | Blood | Single | NLR | 4.79 | 134 | 5 | 1 | 52 | 76 |
| PLR | 37.7 | 97 | 16 | 71 | 4 | 6 | |||||||||
| Zhang,S et al. [17] | 2021 | China | Pro | Preterm(NA),Term Infants(NA) | Blood culture | EOS | Blood | Single | NLR | 3.169 | 124 | 57 | 11 | 17 | 39 |
| PLR | 90.846 | 124 | 48 | 10 | 26 | 40 | |||||||||
| Sumitro.K.R et al. [18] | 2021 | Indonesia | cross-sectional study | Preterm(87.5%),Term Infants(12.5%) | Blood culture | EOS&LOS | Blood | Single | NLR | 2.12 | 104 | 42 | 30 | 10 | 22 |
| Panda.S.K et al. [13] | 2021 | India | Retro | Preterm(100%) | Blood culture | EOS&LOS | Blood | Single | NLR | 1.7 | 93 | 28 | 28 | 13 | 24 |
| Karabulut.B et al. [19] | 2021 | Turkey | Retro | Term Infants(100%) | Clinical diagnosis | EOS | Blood | Single | NLR | 1.42 | 60 | 26 | 5 | 4 | 25 |
| Chen.S.J et al. [20] | 2021 | China | Pro | Preterm(100%), | Clinical diagnosis, blood culture | EOS | Venous cord blood | Single | NLR | NA | 427 | 126 | 51 | 26 | 224 |
| PLR | 50.051 | 176 | 73 | 55 | 16 | 32 | |||||||||
| T. Li, G. Dong et al. [21] | 2020 | China | Retro | Preterm(NA),Term Infants(NA) | Clinical diagnosis | EOS&LOS | Blood | Single | NLR | 1.62 | 925 | 376 | 47 | 361 | 141 |
| Goldberg.O et al. [22] | 2020 | Israel | Retro | Preterm(100%) | Blood culture | LOS | Blood | Single | NLR | 1.5 | 145 | 42 | 19 | 6 | 78 |
| Wilar.R et al. [23] | 2019 | Indonesia | cross-sectional study | Preterm(20%),Term Infants(80%) | Clinical diagnosis, blood culture | EOS | Blood | Single | NLR | 1.24 | 120 | 75 | 2 | 15 | 28 |
| Arcagok, B.C.et al. [14] | 2019 | Turkey | Retro | Term Infants(100%) | Clinical diagnosis, blood culture | EOS | Blood | Single | PLR | 57.70 | 159 | 61 | 2 | 6 | 90 |
| Omran, A. et al. [24] | 2018 | Egypt | Pro | Term Infants(100%) | Clinical diagnosis, blood culture | EOS&LOS | Blood | Single | NLR | 2.7 | 70 | 28 | 15 | 7 | 20 |
| PLR | 73.10 | 70 | 17 | 19 | 18 | 16 | |||||||||
| Can,E. et al. [25] | 2018 | Turkey | Pro | Term Infants(100%) | Clinical diagnosis, blood culture | EOS | Blood | Single | NLR | 6.76 | 122 | 76 | 0 | 2 | 44 |
| PLR | 94.05 | 122 | 76 | 0 | 2 | 44 | |||||||||
| Alkan Ozdemir, S. et al. [26] | 2018 | Turkey | Pro | Preterm(100%) | Clinical diagnosis, blood culture | LOS | Blood | Single | NLR | 1.77 | 127 | 38 | 16 | 14 | 59 |
Pro Prospective, Retro Retrospective, EOS Early onset sepsis, LOS Late-onset sepsis, NA Not available, NLR Neutrophil lymphocyte ratio, PLR Platelet lymphocyte ratio, FN False negative, FP False positive, TN True negative, TP True positive
QUADAS-2 was applied to assess the risk of bias and applicability of included studies, as shown in Fig. 2. Quality of included studies was considered to be acceptable. Risk of bias assessment showed that: for patient selection, 3 studies were graded as “high risk” due to non-randomized or discontinuous selection; For index test, 11 studies were graded as “high risk” due to that the cut-off values applied were not pre-determined; For reference standard, 1 study was graded as “high risk” due to application of other criteria for NS diagnosis. For flow and timing, 8 studies were graded as “high risk” due to that part of the participants were excluded from data analyses. Applicability assessment showed that 4 studies were graded as “high applicability” in the selection of NS children in that the participants were concomitant with no other diseases, 5 studies were graded as “unclear applicability” in index test due to that detailed process of diagnostic test was not reported, and 2 studies were graded as “high applicability” in reference standard due to that the gold standard was not applied.
Fig. 2.
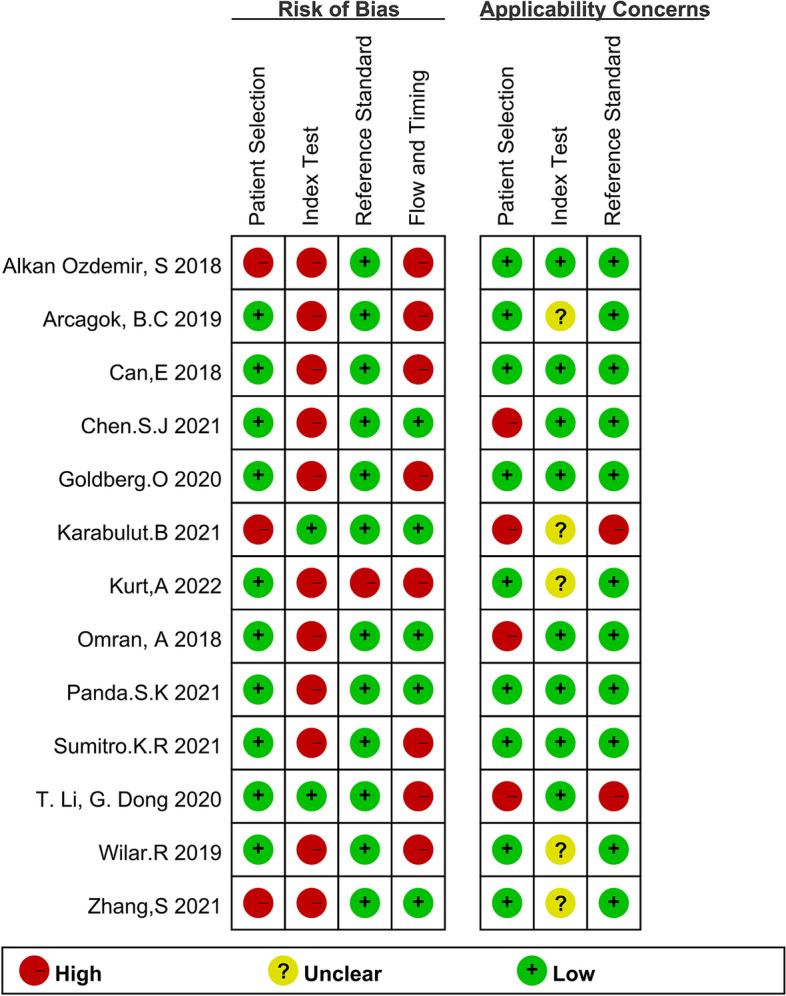
Risk of bias and applicability assessment of included studies
Diagnostic performance of NLR for NS
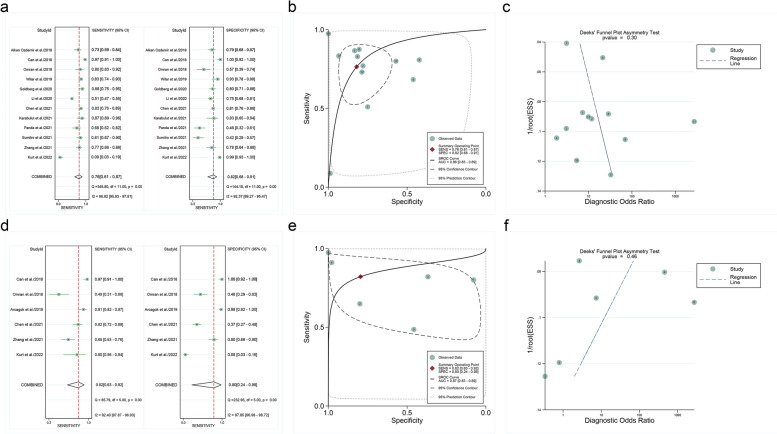
The pooled SEN of NLR was 0.76 (95%CI: 0.61–0.87), with significant heterogeneity (96.82%). The SPE was 0.82 (95%CI: 0.68–0.91), with significant heterogeneity (92.37%). The AUC of NLR was 0.86 (95%CI:0.83–0.89).
Subgroup analysis for EOS showed that the pooled SEN, SPE, and AUC of NLR were 0.87 (95%CI: 0.77–0.93), 0.90 (95%CI: 0.73–0.97) and 0.94 (95%CI: 0.92–0.96), respectively. Detailed results are shown in Fig. 3a and b. The results of subgroup analysis and meta-regression were shown in Table 2.
Fig. 3.
a Pooled SEN and SPE of NLR for diagnosing NS, b NLR symmetrical summary receiver operator characteristic (SROC) curve for included studies, c Deeks’ funnel plot for publication bias, d Pooled SEN and SPE of PLR for diagnosing NS, e PLR symmetrical summary receiver operator characteristic (SROC) curve for included studies, f Deeks’ funnel plot for publication bias
Table 2.
Subgroup analysis of diagnostic performance of NLR
| Covariate/Subgroup | Studies, n | Sensitivity(95%CI) | P-value | Specificity(95%CI) | P-value |
|---|---|---|---|---|---|
| Number of samples | 0.05 | 0.75 | |||
| > 130 | 4 | 0.58[0.31–0.85] | 0.87[0.74-1.00] | ||
| ≤ 130 | 8 | 0.83[0.72–0.95] | 0.78[0.63–0.94] | ||
| Country | 0.59 | 0.60 | |||
| China | 3 | 0.72[0.43-1.00] | 0.79[0.54-1.00] | ||
| Other countries | 9 | 0.78[0.63–0.93] | 0.83[0.71–0.96] | ||
| Study design | 0.24 | 0.75 | |||
| Prospective | 5 | 0.85[0.71–0.99] | 0.83[0.68–0.99] | ||
| Retrospective and Clinical | 5 | 0.61[0.35–0.87] | 0.83[0.67-0.0.98] | ||
| Gold standard | 0.95 | 0.03 | |||
| Blood culture | 4 | 0.80[0.58-1.00] | 0.64[0.37–0.91] | ||
| Clinic, blood culture | 6 | 0.76[0.56–0.96] | 0.91[0.81-1.00] | ||
| Type of Sepsis | 0.01 | 0.11 | |||
| EOS&LOS | 5 | 0.57[0.33–0.80] | 0.72[0.50–0.94] | ||
| EOS | 5 | 0.87[0.76–0.89] | 0.89[0.78-1.00] | ||
| Specimen | 0.94 | 0.68 | |||
| Blood | 11 | 0.76[0.62–0.90] | 0.82[0.70–0.94] | ||
| Venous cord blood | 1 | 0.83[0.48-1.00] | 0.82[0.44-1.00] | ||
| cut-off | 0.54 | 0.04 | |||
| > 3 | 3 | 0.69[0.35-1.00] | 0.96[0.90-1.00] | ||
| ≤ 3 | 8 | 0.78[0.62–0.94] | 0.72[0.57–0.87] |
For all studies on NLR, the median cut off value is 2.12, and then the integer 3 is taken
Subgroup analysis and meta-regression were performed to identify the sources of heterogeneity. Types of sepsis (p = 0.01 for SEN), gold standard (p = 0.03 for SPE), and the cut-off values (p<0.05 for SPE) might be the sources of NLR heterogeneity. Deek’s funnel plot for included studies showed no significant publication bias (Fig. 3c, p = 0.30).
Diagnostic performance of PLR for NS
The pooled SEN of PLR was 0.82 (95%CI:0.63–0.92), with significant heterogeneity (92.40%). The SPE was 0.80 (95%CI:0.24–0.98), with significant heterogeneity (97.85%). The AUC of PLR was 0.87 (95%CI:0.83–0.89).
Subgroup analysis for EOS showed that the pooled SEN, SPE, and AUC of PLR were 0.82 (95%CI: 0.63–0.92), 0.80 (95%CI: 0.24–0.98) and 0.87 (95%CI: 0.83–0.89), respectively. Detailed results are shown in Fig. 3d and e. The results of subgroup analysis and meta-regression were shown in Table 3.
Table 3.
Subgroup analysis of diagnostic performance of PLR
| Covariate/Subgroup | Studies, n | Sensitivity(95%CI) | P-value | Specificity(95%CI) | P-value |
|---|---|---|---|---|---|
| Number of samples | 0.49 | 0.24 | |||
| >130 | 2 | 0.88[0.71-1.00] | 0.84[0.24-1.00] | ||
| ≤ 130 | 4 | 0.78[0.58–0.97] | 0.80[0.26-1.00] | ||
| Country | 0.47 | 0.76 | |||
| China | 2 | 0.74[0.45-1.00] | 0.60[-0.36-1.00] | ||
| Other countries | 4 | 0.85[0.71-1.00] | 0.87[0.51-1.00] | ||
| Study design | 0.65 | 0.10 | |||
| Prospective | 4 | 0.80[0.62–0.98] | 0.87[0.49-1.00] | ||
| Retrospective and Clinical | 2 | 0.87[0.68-1.00] | 0.66[-0.33-1.00] | ||
| Gold standard | 0.41 | 0.31 | |||
| Blood culture | 1 | 0.65[0.17-1.00] | 0.81[-0.09-1.00] | ||
| Clinic, blood culture | 5 | 0.85[0.72–0.98] | 0.79[0.34-1.00] | ||
| Type of Sepsis | 0.07 | 0.48 | |||
| EOS&LOS | 1 | 0.49[0.03–0.94] | 0.46[-0.99-1.00] | ||
| EOS | 5 | 0.86[0.75–0.96] | 0.85[0.50-1.00] | ||
| Specimen | 0.79 | 0.05 | |||
| Blood | 5 | 0.81[0.65–0.97] | 0.86[0.53-1.00] | ||
| Venous cord blood | 1 | 0.82[0.49-1.00] | 0.37[-0.95-1.00] | ||
| cut-off | 0.46 | 0.00 | |||
| > 90 | 2 | 0.88[0.71-1.00] | 0.99[0.92-1.00] | ||
| ≤ 90 | 4 | 0.78[0.59–0.97] | 0.53[-0.10-1.00] |
For all studies on PLR, the median cut off value is 81.793, and then the integer 90 is taken
Subgroup analysis and meta-regression were performed to identify the sources of heterogeneity. The cut-off values (p<0.05 for SPE) might be the source of PLR heterogeneity. Deek’s funnel plot for included studies showed no significant publication bias (Fig. 3f, p = 0.46).
Discussion
Neutrophil count, lymphocyte count, and platelet count are often utilized as clinical indicators in blood analysis, which entails a quick and accessible laboratory investigation [27]. Indicators of NLR and PLR generated from blood analysis have received interest in the study of inflammation-related disorders in recent years [28]. PLR is increased in the inflammatory response as the microcirculation of the body is altered, the permeability of blood vessels is increased, platelets are activated, and a large number of platelets are aggregated, which in turn aggravates the inflammatory response of the body [29]. NLR is considered to be a more sensitive indicator for microbial infection. It rises rapidly after being infected and is often associated with disease severity [30, 31]. There have been increasing studies demonstrating that NLR and PLR would be of clinical significance for the diagnosis of NS [25, 32, 33], and would be associated with the severity and prognosis of the disease. However, it is still unclear which is the better diagnostic value of NLR or PLR for neonatal sepsis. This study has performed meta-analysis for studies evaluating NLR and PLR for the diagnosis of NS, and has comprehensively assessed the diagnostic value of NLR and PLR, so as to provide a reference for clinical early identification of NS. Subgroup analysis for EOS showed that the pooled SEN, SPE, and AUC of NLR were 0.87 (95%CI: 0.77–0.93), 0.90 (95%CI: 0.73–0.97) and 0.94 (95%CI: 0.92–0.96), respectively. Subgroup analysis for EOS showed that the pooled SEN, SPE, and AUC of PLR were 0.82 (95%CI: 0.63–0.92), 0.80 (95%CI: 0.24–0.98) and 0.87 (95%CI: 0.83–0.89), respectively.
This is the first systematic review and meta-analysis comparing the two indicators for NS diagnosis. Data on the participants in the 13 studies were collected and analyzed, and the results showed that the SEN, SPE, and AUC of NLR were 0.76, 0.82, and 0.86, respectively, and those of PLR were 0.82, 0.82, and 0.87, respectively. Both the two indicators have presented great and similar diagnostic accuracy, which indicates that NLR and PLR are of great accuracy in diagnosing NS, and of remarkable value for clinical screening and definite diagnosis of NS. These two indicators can be considered as reliable biomarkers of early-stage NS.
Deek’s funnel plot showed no significant publication bias existing, while there was significant heterogeneity, among included studies. Subgroup analysis and meta-regression showed that for NLR, types of sepsis might be the source of heterogeneity of SEN, whereas the gold standard and the cut-off values might be that of SPE. As for PLR, the cut-off values might be the source of heterogeneity of SPE, while the source of heterogeneity of SEN could not be identified.
The diagnostic cut-off values for NLR and PLR varied across the included studies, which might induce heterogeneity. Specifically, the cut-off values for NLR ranged from 1.24 to 6.76, while that for PLR ranged from 37.7 to 97.4. The differences in cut-off values could be explained by variations in the study population, the type and severity of sepsis, and the laboratory testing methods used to measure NLR and PLR. Furthermore, the optimal cut-off values for NLR and PLR might depend on the context and the diagnostic performance criteria. Future studies should aim to establish standardized cut-off values for NLR and PLR that can be applied across different populations and settings.
This study also has some limitations: firstly, only 13 studies were included in the meta-analysis, which lead to a small sample size. Secondly, all the included studies are retrospective or cross-sectional, more prospective studies in this area are needed to draw more robust conclusions. Lastly, none of the thresholds of PLR or NLR for the research were settled before the diagnosis, which could lead to an overestimation of their diagnostic value.
Conclusion
In summary, this meta-analysis has demonstrated that NLR and PLR would be of great sensitivity and specificity in the diagnosis of NS, and can be used as biomarkers for early diagnosis of NS. These two indicators have shown similarly remarkable diagnostic accuracy in the detection of NS, which indicates that NLR and PLR would be accurate and reliable in the diagnosis of NS. Clinical pediatricians can consider using these two laboratory indicators to diagnose NS, with a view to early detection, early diagnosis and early treatment of NS, so as to improve the cure rate of NS and shorten the treatment cycle. However, the overall risk of bias was high, and significant heterogeneity was identified among the included studies. The results of this study should be interpreted prudently, and the normal or cut-off values and the type of sepsis should be considered. More prospective studies are needed to further support the clinical application of these findings.
Acknowledgements
We would like to thank the researchers and study participants for their contributions.
Authors’ contributions
Conceptualization: Peihui Gong, Yueqin Zhang, Hao Zhou, Xiaoyun Jia, Xinhua Zhang; Methodology: Peihui Gong, Xiaoyun Jia, Xinhua Zhang; Software: Peihui Gong; Data curation: Peihui Gong, Lixia Bai, Xiuhui Li, Yanan Kang, Xiaoyun Jia, Xinhua Zhang; Investigation: Lixia Bai; Resources: Lixia Bai, Xinhua Zhang, Xiuhui Li, Xiaoyun Jia; Supervision: Lixia Bai; Formal analysis: Yueqin Zhang; Funding acquisition: Xiaoyun Jia; Writing - Original Draft/Reviewing and Editing: All authors. The author(s) read and approved the final manuscript.
Funding
The study is approved by Key R & D projects in Shanxi Province [No. 201903D321173].
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shi Y. Expert consensus on diagnosis and treatment of neonatal sepsis (2019 version) Chin J Pediatr. 2019;04:252–7. doi: 10.3760/cma.j.issn.0578-1310.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 2.Pammi M, Abrams SA. Enteral lactoferrin for the treatment of sepsis and necrotizing enterocolitis in neonates. Cochrane Database Syst Rev. 2019;5(5):Cd007138. doi: 10.1002/14651858.CD007138.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu IH, Tsai MH, Lai MY, Hsu LF, Chiang MC, Lien R, Fu RH, Huang HR, Chu SM, Hsu JF. Incidence, clinical features, and implications on outcomes of neonatal late-onset sepsis with concurrent infectious focus. BMC Infect Dis. 2017;17(1):465. doi: 10.1186/s12879-017-2574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223–230. doi: 10.1016/S2213-2600(18)30063-8. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID, III, Hale EC, et al. Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127(5):817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pimenta JM, Ebeling M, Montague TH, Beach KJ, Abell J, O’Shea MT, Powell M, Hulsey TC. A retrospective database analysis of neonatal morbidities to evaluate a Composite Endpoint for Use in Preterm Labor Clinical trials. AJP Rep. 2018;8(1):e25–e32. doi: 10.1055/s-0038-1635097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shane AL, Stoll BJ. Neonatal sepsis: progress towards improved outcomes. J Infect. 2014;68(Suppl 1):24–32. doi: 10.1016/j.jinf.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Dal-Pizzol F, Ritter C. Searching for the Holy Grail: where do we go with the current biomarkers for sepsis? Rev Bras Ter Intensiva. 2012;24(2):117–8. doi: 10.1590/S0103-507X2012000200004. [DOI] [PubMed] [Google Scholar]
- 9.van Engelen TSR, Wiersinga WJ, Scicluna BP, van der Poll T. Biomarkers in Sepsis. Crit Care Clin. 2018;34(1):139–152. doi: 10.1016/j.ccc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Nobre V, Borges I. Prognostic value of procalcitonin in hospitalized patients with lower respiratory tract infections. Rev Bras Ter Intensiva. 2016;28(2):179–89. doi: 10.5935/0103-507X.20160019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martins EC, Silveira LDF, Viegas K, Beck AD, Fioravantti Júnior G, Cremonese RV, Lora PS. Neutrophil-lymphocyte ratio in the early diagnosis of sepsis in an intensive care unit: a case-control study. Rev Bras Ter Intensiva. 2019;31(1):64–70. doi: 10.5935/0103-507X.20190010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djordjevic D, Rondovic G, Surbatovic M, Stanojevic I, Udovicic I, Andjelic T, Zeba S, Milosavljevic S, Stankovic N, Abazovic D, et al. Neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and Mean platelet volume-to-platelet count ratio as biomarkers in critically ill and injured patients: which ratio to choose to Predict Outcome and Nature of Bacteremia? Mediators Inflamm. 2018;2018:3758068. doi: 10.1155/2018/3758068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panda SK, Nayak MK, Rath S, Das P. The utility of the neutrophil-lymphocyte ratio as an early diagnostic marker in neonatal Sepsis. Cureus. 2021;13(1):e12891. doi: 10.7759/cureus.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arcagok BC, Karabulut B. Platelet to lymphocyte ratio in neonates: a predictor of early onset neonatal Sepsis. Mediterr J Hematol Infect Dis. 2019;11(1):e2019055. doi: 10.4084/mjhid.2019.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu Y, Yang Z, Shun F. Bias Risk Assessment Series: (six) diagnostic test. Chin J Epidemiol. 2018;39(04):524–31. [Google Scholar]
- 16.Kurt A, Tosun MS, Altuntaş N. Diagnostic accuracy of complete blood cell count and neutrophil-to-lymphocyte, lymphocyte-to-monocyte, and platelet-to-lymphocyte ratios for neonatal infection. Asian Biomed. 2022;16(1):43–52. doi: 10.2478/abm-2022-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang S, Luan X, Zhang W, Jin Z. Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratio as predictive biomarkers for early-onset neonatal Sepsis. J Coll Physicians Surg Pak. 2021;30(7):821–4. doi: 10.29271/jcpsp.2021.07.821. [DOI] [PubMed] [Google Scholar]
- 18.Sumitro KR, Utomo MT, Widodo ADW. Neutrophil-to-lymphocyte ratio as an alternative marker of neonatal Sepsis in developing countries. Oman Med J. 2021;36(1):e214. doi: 10.5001/omj.2021.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karabulut B, Alatas SO. Diagnostic value of neutrophil to lymphocyte ratio and Mean platelet volume on early onset neonatal Sepsis on term neonate. J Pediatr Intensive Care. 2021;10(2):143–7. doi: 10.1055/s-0040-1715104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SJ, Zheng XX, Jin HX, Chen JH, He TF, Chen CE. Can venous cord blood neutrophil to lymphocyte ratio and platelet to lymphocyte ratio predict early-onset sepsis in preterm infants? Clin Exp Obstet Gynecol. 2021;48(4):828–834. doi: 10.31083/j.ceog4804132. [DOI] [Google Scholar]
- 21.Li T, Dong G, Zhang M, Xu Z, Hu Y, Xie B, Wang Y, Xu B. Association of Neutrophil-Lymphocyte Ratio and the Presence of Neonatal Sepsis. J Immunol Res. 2020;2020:7650713. doi: 10.1155/2020/7650713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldberg O, Amitai N, Chodick G, Bromiker R, Scheuerman O, Ben-Zvi H, Klinger G. Can we improve early identification of neonatal late-onset sepsis? A validated prediction model. J Perinatol. 2020;40(9):1315–1322. doi: 10.1038/s41372-020-0649-6. [DOI] [PubMed] [Google Scholar]
- 23.Wilar R. Diagnostic value of eosinopenia and neutrophil to lymphocyte ratio on early onset neonatal sepsis. Korean J Pediatr. 2019;62(6):217–223. doi: 10.3345/kjp.2018.06723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omran A, Maaroof A, Mohammad MHS, Abdelwahab A. Salivary C-reactive protein, mean platelet volume and neutrophil lymphocyte ratio as diagnostic markers for neonatal sepsis. J Pediatr (Rio J) 2018;94(1):82–87. doi: 10.1016/j.jped.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 25.Can E, Hamilcikan Ş, Can C. The value of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio for detecting early-onset neonatal Sepsis. J Pediatr Hematol Oncol. 2018;40(4):e229–32. doi: 10.1097/MPH.0000000000001059. [DOI] [PubMed] [Google Scholar]
- 26.Alkan Ozdemir S, Arun Ozer E, Ilhan O, Sutcuoglu S. Can neutrophil to lymphocyte ratio predict late-onset sepsis in preterm infants? J Clin Lab Anal. 2018;32(4):e22338. doi: 10.1002/jcla.22338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wyllie DH, Bowler IC, Peto TE. Relation between lymphopenia and bacteraemia in UK adults with medical emergencies. J Clin Pathol. 2004;57(9):950–5. doi: 10.1136/jcp.2004.017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman Vujacic I, Lalic D, Milovanovic T, Dumic I, Krivokapic Z. Combined Diagnostic Efficacy of Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Mean Platelet Volume (MPV) as Biomarkers of Systemic Inflammation in the Diagnosis of Colorectal Cancer. Dis Markers. 2019;2019:6036979. doi: 10.1155/2019/6036979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Chen W, Qian J, Cui Y. Changes in platelet parameters and their clinical significance in patients with acute exacerbation asthma. Chronic Pathematol J. 2019;20(07):972–5. [Google Scholar]
- 30.Che-Morales JL, Cortes-Telles A. [Neutrophil-to-lymphocyte ratio as a serum biomarker associated with community acquired pneumonia] Rev Med Inst Mex Seguro Soc. 2019;56(6):537–43. [PubMed] [Google Scholar]
- 31.Montaldo P, Rosso R, Santantonio A, Chello G, Giliberti P. Presepsin for the detection of early-onset sepsis in preterm newborns. Pediatr Res. 2017;81(2):329–334. doi: 10.1038/pr.2016.217. [DOI] [PubMed] [Google Scholar]
- 32.Aydogan S, Dilli D, Soysal C, Akduman H, Örün U A, Taşar M, Taşoglu I, Zenciroglu A. Role of systemic immune-inflammatory index in early diagnosis of sepsis in newborns with CHD [J]. Cardiol Young. 2022;32(11):1826–32. [DOI] [PubMed]
- 33.Lee JH. Eosinophil count and neutrophil-to-lymphocyte count ratio as biomarkers for predicting early-onset neonatal sepsis. Korean J Pediatr. 2019;62(12):438–439. doi: 10.3345/kjp.2019.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.