Table 1.
Reaction optimization.

| |||
|---|---|---|---|
| entry | Lewis acid | ligand | % yielda |
| 1 | none | none | 0 |
| 2 | 20 mol% Sc(OTf)3 | none | 34 |
| 3 | 20 mol% Mg(OTf)2 | none | 14 |
| 4 | 20 mol% Cu(OTf)2 | none | 8 |
| 5 | 20 mol% Zn(OTf)2 | none | 19 |
| 6 | 20 mol% Fe(OTf)3 | none | 7 |
| 7 | 20 mol% La(OTf)3 | none | 4 |
| 8 | 20 mol% Yb(OTf)3 | none | 7 |
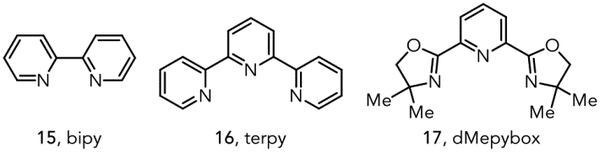
| 9 | 20 mol% Sc(OTf)3 | 30 mol% bipy | 14 |
| 10 | 20 mol% Sc(OTf)3 | 30 mol% terpy | 50 |
| 11 | 20 mol% Sc(OTf)3 | 30 mol% dMepybox | 47 |
| 12 b | 10 mol% Sc(OTf) 3 | 15 mol% terpy | 72 |
| 13c | 20 mol% Sc(OTf)3 | 30 mol% terpy | 0 |
| 14d | 20 mol% Sc(OTf)3 | 30 mol% terpy | 0 |
| 15e | 20 mol% Sc(OTf)3 | 30 mol% terpy | 24 |
| |||
Unless otherwise noted, reactions were conducted using 0.1 mmol 12a, 0.5 mmol 2a, 2 mol% 14, and 2 mL solvent.
Yields were determined by 1H NMR analysis of the crude reaction mixture using CH2Br2 as an internal standard.
0.3 mmol scale, 3 mL CH2Cl2, 25 mol% imidazole, isolated yield.
No photocatalyst.
Run in the dark.
No imidazole.
