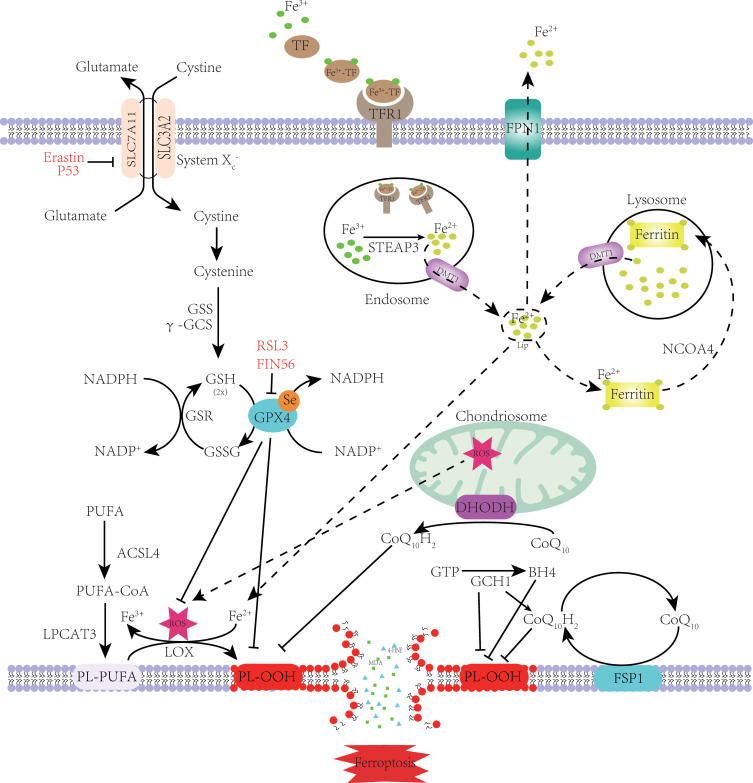
Figure 1.
Mechanism of ferroptosis. PUFA became a part of the cell membrane under the action of ACSL4 and LPCAT3.Through the Fenton reaction and enzymatic reaction, PL-PUFA on the cell membrane undergoes a lipid peroxidation reaction to form PL-OOH. Due to the consumption of PL-PUFA and the toxic effect of peroxidation products, the cell membrane ruptures, eventually causing ferroptosis. Cystine enters the cytoplasm through System Xc- and rapidly transforms into cysteine to participate in GSH synthesis. GPX4 inhibits lipid peroxidation while reducing GSH to GSSG. Lipid peroxidation is also inhibited by FSP1 and DHODH on the cell membrane, which reduce CoQ10 to CoQ10H2. GCH1 can inhibit lipid peroxidation directly or indirectly. When the TF binds to the TFR on the cell membrane, it is endocytosed into the cell. Under the action of STEAP3 and DMT1, Fe2+ enters the cytoplasm and forms labile iron pools. Most Fe2+ is kept in ferritin. When intracellular iron is deficient, ferritin is transported to the lysosomes by NCOA4 to release Fe2+ through iron autophagy. Excess intracellular iron is excreted out of the cell through FPN1. When intracellular iron metabolism is imbalanced, the Fenton reaction and increased iron-containing enzyme activity cause ferroptosis.
Note: Adapted from Zhang X, Ma Y, Lv G, Wang H. Ferroptosis as a therapeutic target for inflammation-related intestinal diseases. Front Pharmacol. 2023;14:1095366.8