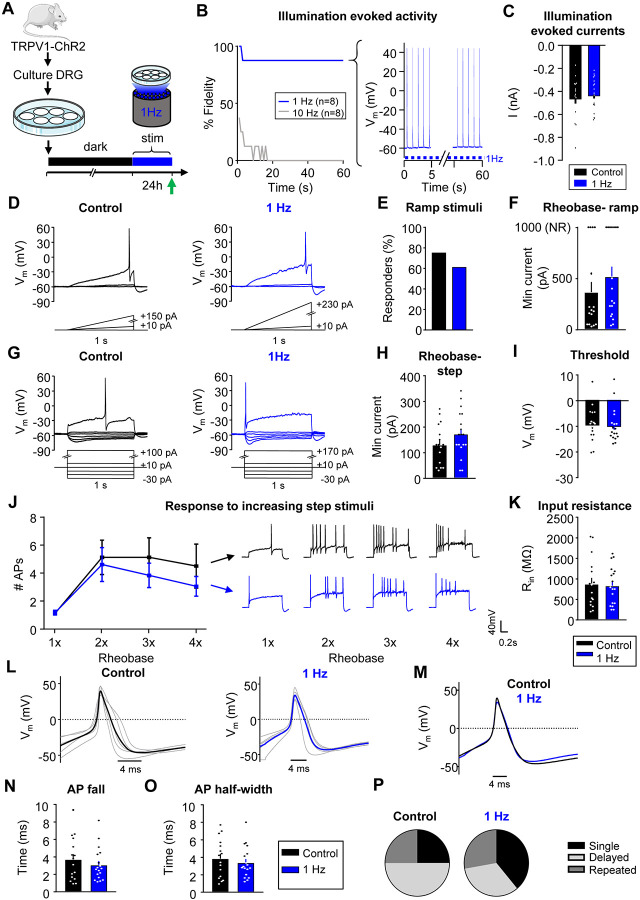
Figure 4: Sustained activity did not alter neuronal excitability in mouse nociceptors.
A) Experimental design for testing homeostatic regulation of intrinsic excitability to sustained activity in cultured small DRG neurons from TRPV1:ChR2 transgenic mice. B) Left: Survival curve of nociceptors exposed to 470nm blue illumination at 1 or 10Hz, 10ms pulses. At 1min, 7/8 cells were still reliably firing with 1Hz stimulation. Right: example trace of a cell firing at 1Hz. C) A 1sec pulse of continuous illumination evoked similar peak currents in control cells kept in the dark (n=16; black) and cells stimulated with 24hrs illumination at 1Hz on an LED array (n=18; blue). Excitability was analyzed as described in Fig 1, but with treatment groups to test a sustained activity stimulus. Comparison of example current clamp traces (D), the proportion of responders (E), and rheobase (F) determined from ramp current injections. Comparison of example current-clamp traces (G), rheobase (H), threshold (I), response to suprathreshold stimuli (J), and input resistance (K) determined from step current injections. Treatment groups: cells kept in the dark (n=16; black), cells exposed to 24h illumination at 1Hz (n=18; blue). All cells were cultured from TRPV1:ChR2 mice. L) Current clamp AP traces from nociceptors treated for 24hrs in the dark (left) or 1Hz stimulation (right). Eight representative action potential traces from each condition (grey) and average (bold color) are overlaid. The first AP at rheobase to step pulses was used. M) Average AP traces are overlaid to show similar AP waveforms. Analysis of AP fall (N) and half-width (O) from all nociceptors. P) Relative proportions of firing pattern subtypes are displayed for each treatment group. Data are represented as mean ± SEM